Semaglutide, manufactured by Novo Nordisk under the brand names Ozempic, Wegovy, and Rybelsus, is FDA-approved for type 2 diabetes and obesity patients. Additionally, it has been tested for other diseases, such as heart and renal diseases. In a recent publication in the NEJM journal, Novo Nordisk released its interim data on the phase 3 trial of semaglutide on MASH (Metabolic Dysfunction Associated Steatohepatitis) patients. MASH is a progressive liver disease with no treatment and poor survival. In 2024, Rezdiffera from Madrigel Pharmaceuticals was the first medicine approved by the FDA for MASH. The details of Rezdiffera’s phase 3 clinical trial can be found here. The following are the details of semaglutide efficacy on MASH steatohepatitis and fibrosis.
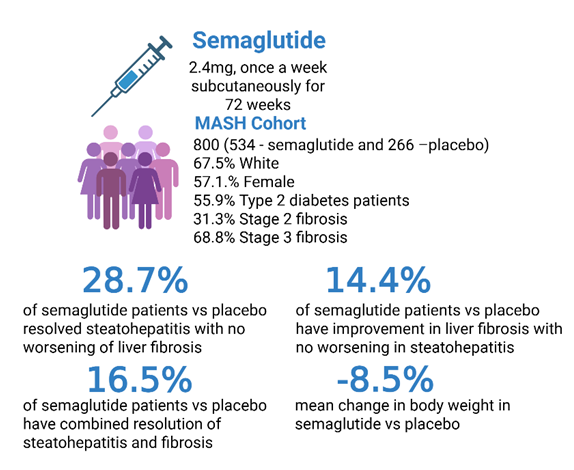
Study details:
1197 patients with MASH and fibrosis stage 2 or 3 were recruited and randomized in a 2:1 ratio of semaglutide or placebo for 240 weeks. In this interim analysis of 72 weeks, data from 800 patients were reported. Semaglutide was given at a dose of 2.4mg in a dose escalation manner, starting from 0.25mg once weekly subcutaneously and increasing to 0.5 mg, 1 mg, 1.7 mg, and 2.4 mg every four weeks.
Primary endpoint outcome:
- 62.9% of patients with semaglutide vs 34.3% of placebo group (difference of 28.7%, 95% CI, 21.1 to 36.2, P<0.001) showed improvement in steatohepatitis without worsening of fibrosis.
- 36.8% in semaglutide vs 22.4% in placebo (14.4% difference, 95% CI, 75 to 21.3; P<0.001) showed a reduction in liver fibrosis score without worsening of steatohepatitis.
Secondary endpoint outcome:
- 32.7% of semaglutide vs 16.1% in placebo reported combined resolution of steatohepatitis and reduction in liver fibrosis (difference of 16.5%; 95% CI, 10.2 to 22.8; P<0.001).
- Mean change in body weight was -10.5% in the semaglutide group vs -2% in the placebo (difference of -8.5%, 95% CI, -9.6 to -7.4; P<0.001).
- The mean change in bodily pain score was not statistically different.
Safety
88% of semaglutide patients maintained the target dose of 2.4 mg until 72 weeks. 86.3% of semaglutide and 79.7% of placebo patients reported an adverse event. Adverse events leading to trial discontinuation occur in 2.6% of semaglutide patients vs 3.3% in placebo. The most common adverse events were gastrointestinal for both groups such as nausea, diarrhea, constipation, and vomiting.
Limitations of the study
- Small number of Black patients.
- Lack of data on biomarkers of alcohol consumption, genetic polymorphisms as a determinant of therapeutic response, and change in body composition during the therapy.
- The number of lean patients was too few to make any definitive conclusion.
Dr. Vinny Negi, Ph.D,
Disclaimer
The editors take care to share authentic information. In case of any discrepancies please write to newsletter@medness.org
The sponsors do not have any influence on the nature or kind of the news/analysis reported in MedNess. The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of MedNess. Examples of analysis performed within this article are only examples. They should not be utilized in real-world analytic products as they are based only on very limited and dated open-source information. Assumptions made within the analysis are not reflective of the position of anyone volunteering or working for MedNess. This blog is strictly for news and information. It does not provide medical advice, diagnosis or treatment nor investment suggestions. This content is not intended to be a substitute for professional medical advice, diagnosis, or treatment. Always seek the advice of your physician or another qualified health provider with any questions you may have regarding a medical condition. Never disregard professional medical advice or delay in seeking it because of something you have read on this website.
MedNess is a part of STEMPeers® which is a 501(c)(3) organization registered in PA as PhD Career Support Group. The organization helps create a growing network of STEM scientists that is involved in peer-to-peer mentoring and support.



