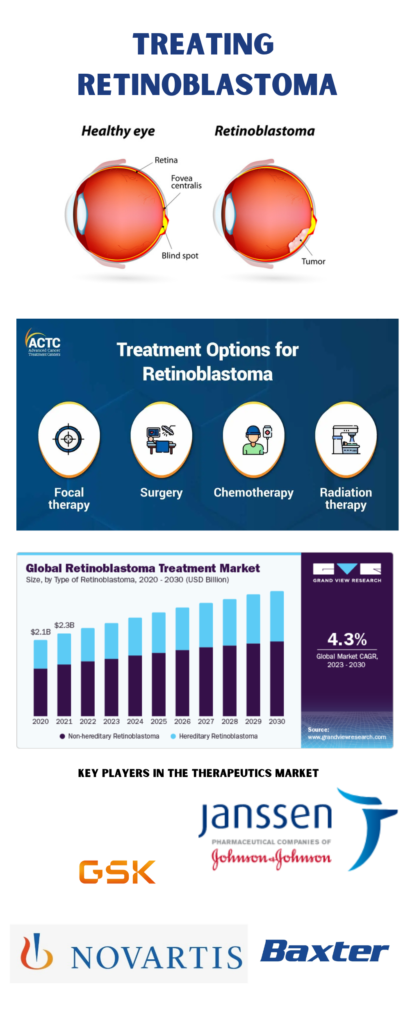
Retinoblastoma is a rare and aggressive form of eye cancer that primarily affects young children. Treatment approaches depend on whether the cancer is confined to the eye (intraocular) or has spread beyond it (extraocular), as well as the stage and recurrence of the disease. Alongside traditional therapies, new treatment options are being explored to address the significant unmet needs in pediatric cancer care. There are six main treatment methods commonly used to treat retinoblastoma:
- Cryotherapy: This technique uses extreme cold to freeze and destroy cancer cells. It is typically employed for small tumors located near the front of the eye.
- Thermotherapy: Heat is applied, often through lasers, to target and destroy cancer cells. It may be used alone for small tumors or in combination with chemotherapy for larger ones.
- Chemotherapy: Chemotherapy drugs are administered either systemically or regionally to target cancer cells.
- Systemic chemotherapy is given intravenously or orally, allowing the drugs to circulate throughout the body and shrink tumors, sometimes avoiding the need for surgery.
- Regional chemotherapy delivers chemotherapy directly to the affected area, such as the eye, through techniques like ophthalmic artery infusion or intravitreal chemotherapy.
- Radiation Therapy: High-energy radiation is used to destroy cancer cells. This can be delivered through external-beam radiation or plaque radiotherapy, where a small radioactive plaque is placed on the eye to target localized tumors.
- High-Dose Chemotherapy with Stem Cell Rescue: This involves using high doses of chemotherapy to eliminate cancer cells, followed by a stem cell transplant to restore the body’s blood-forming cells.
- Surgery (Enucleation): If preserving the eye is not possible, the affected eye may be removed (enucleation), and an artificial eye is fitted afterward. The removed tissue is then examined for signs of cancer spread.
In addition to conventional treatments, **VCN-01**, an oncolytic adenovirus developed by Theriva Biologics, shows promise as a new therapy for retinoblastoma. This treatment has received both “orphan drug” and “rare pediatric drug” designations from the FDA, underscoring the urgent need for new options in this field.
VCN-01 is a systemic, selective oncolytic virus that targets and replicates within tumor cells while degrading the surrounding tumor stroma. This process not only helps to directly destroy cancer cells but also enhances chemotherapy effectiveness and boosts the immune response against the tumor. Initial results from a phase 1 clinical trial for intraocular retinoblastoma (NCT03284268) have been promising. In the trial, pediatric patients aged 1-12 years with chemotherapy- or radiotherapy-resistant intraocular retinoblastoma received two intravitreal injections of VCN-01, 14 days apart. The treatment was well tolerated, with only mild side effects and no dose-limiting toxicities. Some patients experienced ocular inflammation, which was managed with anti-inflammatory drugs. Four patients showed positive responses, including a reduction in vitreous seed density, and three were able to avoid eye removal, with one patient preserving their eye for four years post-treatment.
The potential of VCN-01, combined with its success in other cancers, offers new hope for children with advanced or refractory retinoblastoma, reducing the need for enucleation and improving overall outcomes. The addition of novel therapies like VCN-01, alongside traditional treatments, is advancing the treatment landscape for retinoblastoma. Regular follow-up care and participation in clinical trials are vital for further improving treatment outcomes.
The global retinoblastoma treatment market, valued at USD 2.42 billion in 2022, is expected to grow at a compound annual growth rate (CAGR) of 4.26% from 2023 to 2030. This growth is driven by factors such as the rising incidence of retinoblastoma, advancements in medical technology, and increased research and development in oncology, alongside growing funding and government initiatives supporting the disease. Read the VCN-01story here
Sources
- https://www.aao.org/eye-health/diseases/what-is-retinoblastoma
- https://wechope.org/retinoblastoma/resources/retinoblastoma-communities-social-media/
- https://rarediseases.org/rare-diseases/retinoblastoma/
- https://www.cancer.gov/types/retinoblastoma/patient/retinoblastoma-treatment-pdq
- Figure – https://actchealth.com/blogs/retinoblastoma-101
- Figure – https://www.grandviewresearch.com/industry-analysis/retinoblastoma-treatment-market-report
- Figure – https://medlineplus.gov/genetics/condition/retinoblastoma/
Dr. Malini Gupta, Ph.D.
Disclaimer
The editors take care to share authentic information. In case of any discrepancies please write to newsletter@medness.org
The sponsors do not have any influence on the nature or kind of the news/analysis reported in MedNess. The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of MedNess. Examples of analysis performed within this article are only examples. They should not be utilized in real-world analytic products as they are based only on very limited and dated open-source information. Assumptions made within the analysis are not reflective of the position of anyone volunteering or working for MedNess. This blog is strictly for news and information. It does not provide medical advice, diagnosis or treatment nor investment suggestions. This content is not intended to be a substitute for professional medical advice, diagnosis, or treatment. Always seek the advice of your physician or another qualified health provider with any questions you may have regarding a medical condition. Never disregard professional medical advice or delay in seeking it because of something you have read on this website.
MedNess is a part of STEMPeers® which is a 501(c)(3) organization registered in PA as PhD Career Support Group. The organization helps create a growing network of STEM scientists that is involved in peer-to-peer mentoring and support.



