There is currently no cure for multiple myeloma; however, there are numerous treatment options available to effectively manage the disease, with new ones arising from time to time.
The primary objective of treatment for multiple myeloma is to
- Alleviate symptoms and minimize the number of myeloma cells in the body in a safe and expeditious manner
- Reduce the quantity of M protein (as determined by serum protein electrophoresis) or light chains (as determined by the free light chain test) to the lowest achievable level.
- Eliminate myeloma cells from the bone marrow (as determined by minimal residual disease [MRD] testing)
- Improve the quality of life with the least amount of treatment-related side effects conceivable
- Extension of overall survival.
Induction therapy is the standard of care for newly diagnosed multiple myeloma, and it is followed by maintenance therapy and an autologous stem cell transplant (ASCT – if eligible). Collectively, these therapies are treated as a single line of treatment. A three-drug or four-drug combination regimen is typically administered over three to four cycles, each of which lasts three to four weeks, in induction therapy, or front-line therapy. Depending upon eligibility, patients may elect to complete induction therapy and contemplate a transplant later. In the event of a transplant, patients typically undergo induction therapy, stem cell collection and storage, high dose melphalan chemotherapy, and ASCT. This is succeeded by consolidation and/or maintenance therapy. Maintenance (continuous) therapy is administered subsequent to induction and autologous stem cell transplantation, or in certain patients ineligible for ASCT – directly post induction therapy. Maintenance therapy can enhance survival and extend the duration of remission; however, it is also linked to treatment-related adverse effects.
Commonly used drug regimens for Multiple myeloma involves proteasome inhibitors, immunomodulatory medicines, antibodies, CAR-T cell therapy and B-cell maturation antigen (BCMA)–targeted treatments, as well as steroids and chemotherapy. Each works differently to regulate and kill multiple myeloma cells.
One of the latest approvals in the field, FDA approved daratumumab and hyaluronidase-fihj (Darzalex Faspro), Janssen Research & Development, LLC) in combination with bortezomib, lenalidomide, and dexamethasone for induction and consolidation in newly diagnosed multiple myeloma patients eligible for autologous stem cell transplant on July 30, 2024. PERSEUS (NCT03710603), an open-label, randomized, active-controlled trial in people with newly diagnosed multiple myeloma who were candidates for ASCT, looked at how well the treatment worked. Patients younger than 70 years old were the only ones who could sign up. The 709 patients were split into two groups: 355 were given Darzalex Faspro with bortezomib, lenalidomide, and dexamethasone (Darzalex Faspro-VRd), and 354 were given bortezomib, lenalidomide, and dexamethasone (VRd). The main measure of how well the treatment worked was progression-free survival (PFS), which was decided by an outside review group using criteria set by the International Myeloma Working Group (IMWG).
The PERSEUS study show that the Darzalex Faspro-VRd arm has a longer PFS than the VRd arm. However, neither arm hit the median PFS. Given Darzalex Faspro-VRd treatment, the chance of getting worse or dying is 60% lower than with VRd alone (HR [95% CI]: 0.40 [0.29–0.57]; p-value < 0.0001).Most of the bad responses (≥20%) are shown to be peripheral neuropathy, fatigue, edema, fever, upper respiratory infection, constipation, diarrhea, insomnia, musculoskeletal pain, and rash. The right amount of Darzalex Faspro to take is 1,800 mg/30,000 units, which is made up of 1,800 mg of daratumumab and 30,000 units of hyaluronidase.
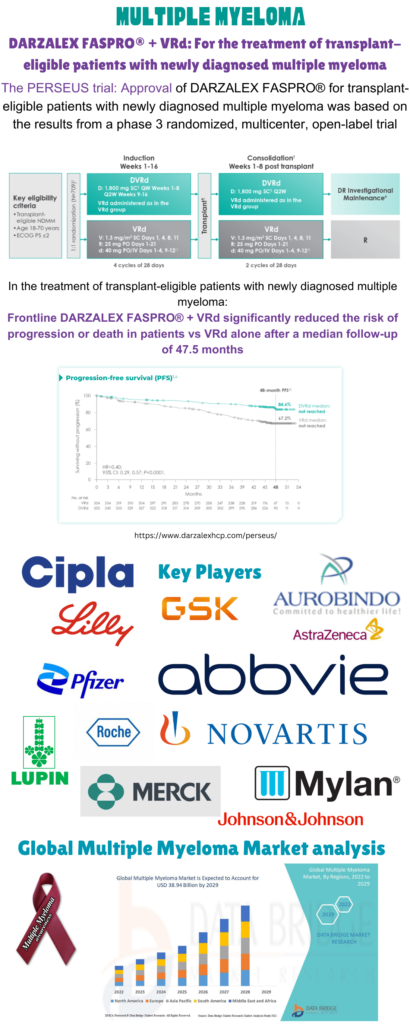
Image: Latest Approved PERSEUS Trail, Key Players and Market Analysis of Multiple Myeloma
Data Bridge Market Research says that the multiple myeloma market was worth USD 23.53 billion in 2021 and will be worth USD 38.94 billion by 2029, growing at a compound annual growth rate (CAGR) of 6.50% from 2022 to 2029.
Dr. Malini Gupta, Ph.D.
Sources
- https://themmrf.org/diagnosis-and-treatment/clinical-trials-and-emerging-therapies/
- https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-daratumumab-and-hyaluronidase-fihj-bortezomib-lenalidomide-and-dexamethasone-multiple
- https://www.darzalex.com/dvrd/#
- Figure elements from : https://www.darzalexhcp.com/perseus/
- Figure elements from : Respective company websites
- Figure elements from ://www.databridgemarketresearch.com/reports/global-multiple-myeloma-market
- https://www.janssen.com/darzalex-faspror-daratumumab-and-hyaluronidase-fihj-based-quadruplet-regimen-approved-us-patients
Disclaimer
The editors take care to share authentic information. In case of any discrepancies please write to newsletter@medness.org
The sponsors do not have any influence on the nature or kind of the news/analysis reported in MedNess. The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of MedNess. Examples of analysis performed within this article are only examples. They should not be utilized in real-world analytic products as they are based only on very limited and dated open-source information. Assumptions made within the analysis are not reflective of the position of anyone volunteering or working for MedNess. This blog is strictly for news and information. It does not provide medical advice, diagnosis or treatment nor investment suggestions. This content is not intended to be a substitute for professional medical advice, diagnosis, or treatment. Always seek the advice of your physician or another qualified health provider with any questions you may have regarding a medical condition. Never disregard professional medical advice or delay in seeking it because of something you have read on this website.
MedNess is a part of STEMPeers® which is a 501(c)(3) organization registered in PA as PhD Career Support Group. The organization helps create a growing network of STEM scientists that is involved in peer-to-peer mentoring and support.



