Kennedy’s disease, also known as Spinal and Bulbar Muscular Atrophy (SBMA), first identified over two decades ago due to a mutation that expands a CAG trinucleotide repeat in the androgen receptor gene, results in an androgen receptor protein with an extended polyglutamine sequence. These disorders are characterized by protein aggregation, mitochondrial dysfunction, and transcriptional abnormalities.
Efforts to develop effective treatments for Kennedy’s disease (SBMA) have faced challenges despite years of research. Although attempts to treat SBMA by reducing androgen levels have been made, clinical trials have yielded only modest results and often face complications from the side effects of long-term androgen withdrawal. While hormone suppression strategies have shown limited success in slowing disease progression in patients, they have been highly effective in mouse models, where chemical or surgical castration can even reverse the disease. Recent insights into SBMA pathology include the observation that androgen receptor (AR) knockdown shows promise in preclinical studies, even though hormone suppression has minimal impact on disease progression once symptoms appear. Recent research has shifted focus to the mutant androgen receptor protein itself. Misfolded versions of this protein are known to be toxic to motor neurons, leading to ongoing efforts to either prevent their formation or promote their degradation. Despite progress in understanding the role of nuclear localization and protein misfolding in the disease, effective treatments have remained elusive.
Two of the latest developments in the therapeutic area against Kennedy Disease has been discussed here:
On May 16, 2024, Avenue Therapeutics announced the successful completion of the final patient visit in its Phase 1b/2a clinical trial NCT05517603. evaluating AJ201 for Kennedy Disease. This milestone marked a significant step in the development of AJ201, a promising new treatment option for a disease that currently has no approved therapies. The 12-week, multicenter, randomized, double-blind trial enrolled 25 patients who were randomly assigned to receive either AJ201 (600 mg/day) or a placebo in a 3:1 ratio. The primary objective of the trial was to assess the safety and tolerability of AJ201 in patients with clinically and genetically confirmed SBMA. Secondary objectives included pharmacokinetic and pharmacodynamic assessments, specifically measuring changes in mutant androgen receptor (AR) protein levels and gene expression in muscle. Exploratory goals involved evaluating changes in muscle and fat composition through MRI scans, which could indicate potential long-term clinical benefits. AJ20 demonstrated strong preclinical results and favorable safety data from previous studies involving healthy volunteers. These findings underscored the potential of AJ201 to address SBMA, a rare and debilitating condition that currently lacks effective treatments. Avenue Therapeutics plans to release topline results from the Phase 1b/2a (NCT05517603). trial by mid-2024.
Nido Biosciences (Nido Bio), a clinical-stage company focused on developing precision medicines for severe neurological disorders, announced a new publication in the Journal of Clinical Investigation Insight on July 8, 2024 titled “Protein Biomarker Signature in Patients with Spinal and Bulbar Muscular Atrophy,”. This revealed a novel and robust set of fluid protein biomarkers linked to Kennedy disease. This research, conducted by teams from Nido Bio, University College London (UCL), the National Institutes of Health (NIH), and the University of Oxford, identified 40 reproducible SBMA-associated proteins from nearly 3,000 measured in plasma and serum samples from patients and matched healthy controls. Notably, two of these proteins were associated with reduced survival and body weight in a mouse model of SBMA. These biomarkers could enhance the tracking of disease progression and serve as early indicators of treatment effects in clinical trials. In parallel with this research progress, Nido Biosciences is advancing its lead therapeutic candidate, NIDO-361, currently undergoing Phase 2 trials. NIDO-361 is a novel small molecule designed to target a specific site on the androgen receptor, aiming to correct transcriptional dysregulation and restore normal cell function.
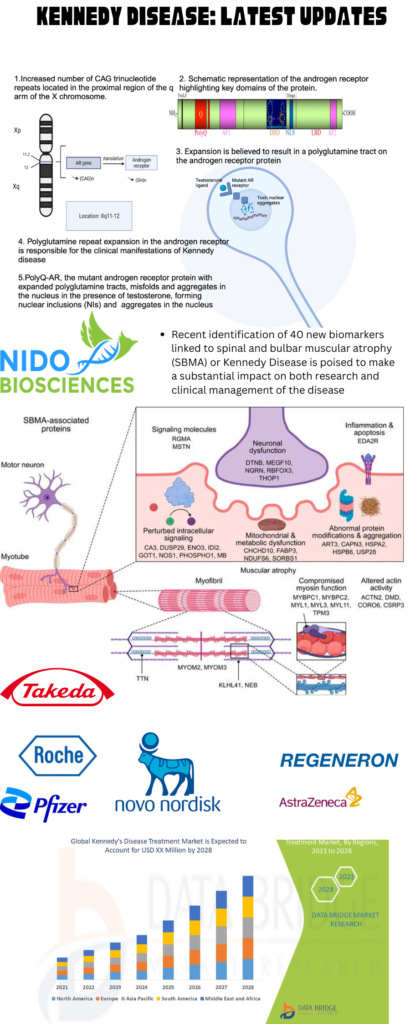
Image: Latest Updates, Key players and Market for treating Kennedy Disease; Image Source: Malini Gupta
Overall, advancing the understanding of Kennedy requires continued research across multiple fronts to identify effective treatments and therapeutic strategies. The Kennedy’s disease treatment market is projected to grow at a rate of 5.50% during the forecast period from 2021 to 2028, according to a report by Data Bridge Market Research. The rising incidence of spinal bulbar muscular atrophy is driving the expansion of the Kennedy’s disease treatment market. Click to find out the biomarker story here.
Dr. Malini Gupta, Ph.D.
Sources
- Image schematic source: https://insight.jci.org/articles/view/176383 :
- https://www.orpha.net/en/disease/detail/481
- https://kennedysdisease.org/
- https://rarediseases.info.nih.gov/diseases/6818/kennedy-disease
- https://rarediseases.org/rare-diseases/kennedy-disease/
- https://www.preventiongenetics.com/searchTests?val=SBMA
- https://nidobio.com/
- https://www.databridgemarketresearch.com/reports/global-kennedys-disease-treatment-market
- https://avenuetx.com/aj201/
Disclaimer
The editors take care to share authentic information. In case of any discrepancies please write to newsletter@medness.org
The sponsors do not have any influence on the nature or kind of the news/analysis reported in MedNess. The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of MedNess. Examples of analysis performed within this article are only examples. They should not be utilized in real-world analytic products as they are based only on very limited and dated open-source information. Assumptions made within the analysis are not reflective of the position of anyone volunteering or working for MedNess. This blog is strictly for news and information. It does not provide medical advice, diagnosis or treatment nor investment suggestions. This content is not intended to be a substitute for professional medical advice, diagnosis, or treatment. Always seek the advice of your physician or another qualified health provider with any questions you may have regarding a medical condition. Never disregard professional medical advice or delay in seeking it because of something you have read on this website.
MedNess is a part of STEMPeers® which is a 501(c)(3) organization registered in PA as PhD Career Support Group. The organization helps create a growing network of STEM scientists that is involved in peer-to-peer mentoring and support.



