I-cell disease or Mucolipidosis Type II (MLII) is a devastating rare genetic disorder characterized by severe multisystemic involvement and a limited lifespan. It is a severe lysosomal storage disorder caused by mutations in the GNPTAB gene located on chromosome 12q23.2. This gene encodes the alpha and beta subunits of N-acetylglucosamine-1-phosphotransferase (GlcNAc-1-phosphotransferase), which plays a crucial role in the proper processing and packaging of lysosomal enzymes within the Golgi apparatus.
ML II and III present with a spectrum of severity, with ML II being more severe and early-onset compared to ML III, which manifests with milder symptoms and a later onset. The
variability in clinical presentation underscores the need for accurate genetic characterization to guide prognosis and management decisions. Despite significant advances, challenges remain in correlating genotype with phenotype due to the complex nature of mutations and their impact on enzyme function.
Diagnosis
Diagnosis relies on biochemical assays and genetic testing, while treatment options remain supportive with a focus on managing symptoms and improving quality of life. Biochemical analysis serves as a cornerstone in confirming the clinical diagnosis of I-cell disease or MLII which involves measuring lysosomal enzyme activities in patient samples, particularly arylsulfatase A and β-glucuronidase, which are transported to lysosomes via the M6P-dependent pathway. Diagnostic criteria typically include a significant increase (at least tenfold) in plasma levels of these enzymes, reflecting their mis localization and accumulation due to the deficiency of GlcNAc-1-phosphotransferase activity. Direct measurement of UDP-GlcNAc-1-phosphotransferase activity remains the gold standard for differentiating ML from other LSDs, although it’s complex and not widely available. Biochemical analyses using single-chain antibody fragments that recognize mannose-6-phosphate (M6P) are also employed to distinguish ML from MPSs. Molecular genetics has emerged as a pivotal diagnostic tool due to its precision by targeted sequencing of the GNPTAB gene to identify specific mutations that impair enzyme function. Mutational analysis of GNPTAB and GNPTG genes is crucial to confirm the subtype of ML (II, III α/β, or III γ), highlighting the importance of genetic testing in clinical practice.
Treatment Options
The lack of any approved treatments for I-cell disease highlights the challenges associated with developing effective therapies for rare diseases. Current treatments primarily focus on supportive care and symptom management. Physiotherapeutic interventions tailored to individual patient needs are crucial for maintaining mobility and preventing joint complications. Surgical interventions such as joint replacements are considered for severe cases where bone and joint deformities significantly impair quality of life. Bisphosphonate therapy has shown promise in managing skeletal manifestations by inhibiting bone resorption, although its long-term efficacy and safety in ML require further study.
Research into novel therapeutic modalities like gene therapy, pharmacological chaperones, and substrate reduction therapy holds promise for improving outcomes in ML patients. Experimental therapies like hematopoietic stem cell transplantation (HSCT) and enzyme replacement therapy (ERT) are being explored as potential treatments for ML. HSCT aims to restore enzyme activity through donor-derived hematopoietic cells, potentially slowing disease progression and improving outcomes. ERT, successful in other LSDs, faces challenges in ML due to the nature of the defective enzyme being a membrane protein, necessitating innovative approaches such as gene therapy or pharmacological chaperones to enhance enzyme function or stability Bone marrow transplantation has been explored in a limited number of patients with I-cell disease or ML II. Initial findings suggested that lysosomal enzyme levels may return to normal in some cases post-transplantation, however, recent research indicates that bone marrow transplantation may not provide sufficient therapeutic benefit for this condition.
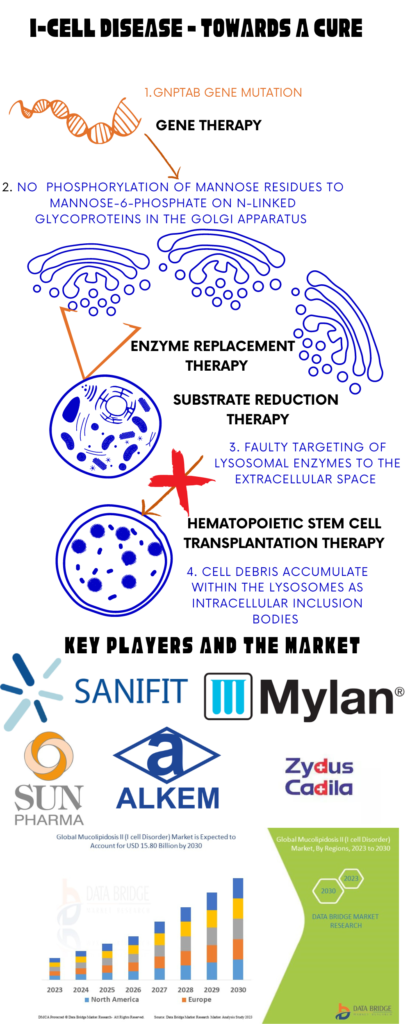
Image I: Treatment Options, Key Players and Market for I-Cell Disease; Image Source: Malini Gupta
While significant strides have been made in understanding the pathophysiology and genetics of I-cell disease, challenges in diagnosis and treatment persist. Advances in molecular genetics have revolutionized diagnostic accuracy, allowing for personalized approaches to patient management.
According to Data Bridge Market Research, the global market for I-cell disease, valued at USD 12.80 billion in 2022, is projected to grow to USD 15.80 billion by 2030. The market is anticipated to achieve a compound annual growth rate (CAGR) of 3.9% from 2023 to 2030. Read more on I-cell disease and other Mucolipidosis cure strategies.
Dr. Malini Gupta, Ph.D.
Sources
- https://www.medicinenet.com/inclusion-cell_i-cell_disease/article.htm
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6824887/
- https://www.frontiersin.org/journals/pediatrics/articles/10.3389/fped.2023.1199489/full
- https://www.databridgemarketresearch.com/reports/global-mucolipidosis-ii-i-cell-disorder-market
Disclaimer
The editors take care to share authentic information. In case of any discrepancies please write to newsletter@medness.org
The sponsors do not have any influence on the nature or kind of the news/analysis reported in MedNess. The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of MedNess. Examples of analysis performed within this article are only examples. They should not be utilized in real-world analytic products as they are based only on very limited and dated open-source information. Assumptions made within the analysis are not reflective of the position of anyone volunteering or working for MedNess. This blog is strictly for news and information. It does not provide medical advice, diagnosis or treatment nor investment suggestions. This content is not intended to be a substitute for professional medical advice, diagnosis, or treatment. Always seek the advice of your physician or another qualified health provider with any questions you may have regarding a medical condition. Never disregard professional medical advice or delay in seeking it because of something you have read on this website.
MedNess is a part of STEMPeers® which is a 501(c)(3) organization registered in PA as PhD Career Support Group. The organization helps create a growing network of STEM scientists that is involved in peer-to-peer mentoring and support.



