It is known that high consumption of artificial sweeteners like aspartame can increase the risk of cardiovascular events but how it actually contributes to the disease is not clear. In the present study published in Cell Metabolism, the authors demonstrated its molecular mechanism in mice. They showed that an aspartame-rich diet in mice increased blood insulin in a glucose-independent manner by stimulating the vagus nerve. The high insulin further increased chemokine CX3CR1 in aortic endothelial cells that attracted monocytes/macrophages forming plaques leading to cardiovascular diseases.
Background
Aspartame is one of the most common low-caloric artificial sweeteners used in various drinks and food products. Its excess consumption is associated with various diseases such as obesity, type 2 diabetes mellitus, cancer, cardiovascular disease, allergies, neurological disorders, and behavior disorders. However, the exact mechanism is not clear. In this study, the authors have investigated the effect of excess aspartame in causing atherosclerotic plaques which are the fat deposits in the arteries causing them to narrow and stiff leading to cardiovascular diseases.
Molecular mechanism
The ApoE-/- mice on a high aspartame diet increased their blood insulin levels leading to plaque formation. Apolipoprotein E (Apo E) is required for the transportation of lipids, cholesterol, and fatty acids throughout the body. Its absence reduces the ability of mice to clear cholesterol from blood and makes them susceptible to atherosclerosis. Aspartame stimulates duodenum cells which further activates the vagus nerve to increase blood insulin levels. The surgical removal of the vagus nerve prevented this increase in insulin and atherosclerotic plaque formation, indicating its significance. High insulin led to increased chemokine CX3CR1 levels in aortic endothelial cells which attracted monocytes/macrophages and differentiated them into M1 macrophages. M1 macrophages are the pro-inflammatory macrophages that initiate the plaque formation. The deletion of the CX3CL1 receptor – CX3CR1 in ApoE-/- mice inhibited the plaque formation induced by aspartame. Thus, CX3CL1-CX3CR1 dependent macrophage-endothelial cell crosstalk is required for aspartame to cause atherosclerotic plaque formation.
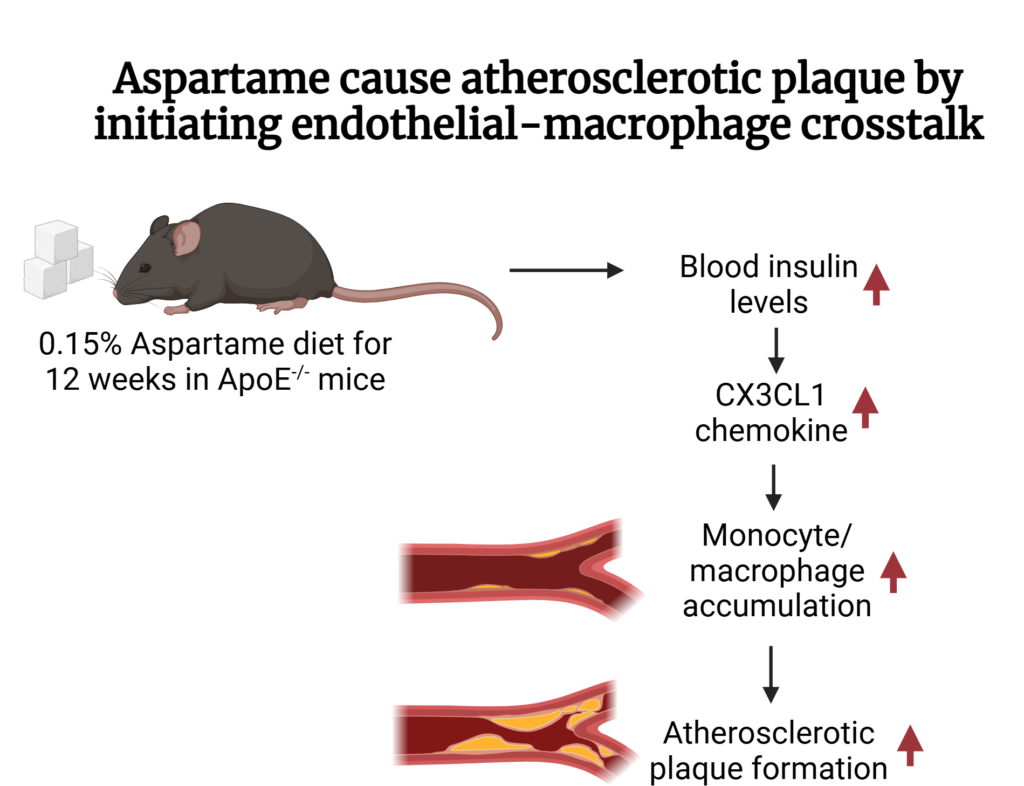
Image 1: Molecular Mechanism Involved: Aspartame causes atherosclerotic plaque by initiating Endothelial- Macrophage cross talk; Image created in BioRender
Limitations of the study
- All the experiments were performed in ApoE-/- knockout mice model, as these mice are susceptible to develop atherosclerosis and the molecular analysis can be done quickly. However, it would be interesting to determine the effect of aspartame on wild-type mice.
- In monkeys, they observed that aspartame increased insulin levels, similar to sucrose consumption, making it difficult to distinguish between the effect of aspartame and sucrose. Furthermore, they did not study plaque formation in monkeys, limiting its clinical relevance.
- In addition to aspartame, there are other artificial sweeteners, which may or may not have a similar mechanism as aspartame. This needs to be further investigated.
- This is not the only mechanism, there might be other mechanisms that contribute to aspartame-induced atherosclerotic plaque formation.
- Lastly, human study is needed to obtain definite conclusions.
Dr. Vinny Negi, Ph.D,
Disclaimer
The editors take care to share authentic information. In case of any discrepancies please write to newsletter@medness.org
The sponsors do not have any influence on the nature or kind of the news/analysis reported in MedNess. The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of MedNess. Examples of analysis performed within this article are only examples. They should not be utilized in real-world analytic products as they are based only on very limited and dated open-source information. Assumptions made within the analysis are not reflective of the position of anyone volunteering or working for MedNess. This blog is strictly for news and information. It does not provide medical advice, diagnosis or treatment nor investment suggestions. This content is not intended to be a substitute for professional medical advice, diagnosis, or treatment. Always seek the advice of your physician or another qualified health provider with any questions you may have regarding a medical condition. Never disregard professional medical advice or delay in seeking it because of something you have read on this website.
MedNess is a part of STEMPeers® which is a 501(c)(3) organization registered in PA as PhD Career Support Group. The organization helps create a growing network of STEM scientists that is involved in peer-to-peer mentoring and support.



