Gaucher disease results from mutations in the GBA gene, disrupting the breakdown of glucocerebroside and causing its accumulation, leading to cellular damage. Efforts to repair this faulty “switch” have long been pursued in medical research. This disease though currently incurable, can be managed effectively with available therapies. Type 1 Gaucher patients can lead full lives, with some not requiring treatment if asymptomatic. For those needing treatment, options include Enzyme Replacement Therapy (ERT) and Substrate Reduction Therapy (SRT).
ERT involves infusions of the deficient enzyme glucocerebrosidase, with three licensed options: Cerezyme©, VPRIV©, and Elelyso©. SRT, an oral therapy, reduces fatty substance buildup in cells. Zavesca© and Cerdelga© are the licensed SRT products. While these therapies alleviate symptoms, they don’t provide a cure. Treatment decisions should be guided by specialist advice, as not all therapies are suitable for every patient. Enzyme Replacement Therapy (ERT) has been a cornerstone, effectively managing symptoms by replacing the deficient enzyme glucocerebrosidase. Recently, substrate reduction therapy has been explored for type 3 Gaucher disease. The emergence of gene modification treatments offers hope for a cure, with gene therapy clinical trials on the horizon. Dr. Neal Weinreb emphasizes the importance of informed decision-making for patients considering participation in these trials, which aim to broaden treatment options and improve understanding of the disease.
Freeline Therapeutics unveiled encouraging clinical data from its Phase 1/2 GALILEO-1 trial of FLT201, an adeno-associated virus (AAV) gene therapy candidate for Gaucher disease. The data, presented at the American Society of Gene and Cell Therapy (ASGCT) 27th Annual Meeting, showcased substantial reductions in glucosylsphingosine (lyso-Gb1), a key predictor of clinical response, in patients with persistently high levels despite years of treatment with current therapies. FLT201 showed promising improvements in bone marrow burden and fatigue, with a favorable safety profile observed. Notable findings included robust expression of plasma GCase, cellular uptake of GCase from plasma, and hematological improvements post withdrawal of prior therapies. Emerging data demonstrated reductions in bone marrow burden and clinically meaningful fatigue improvement. FLT201 was granted RMAT designation by the US FDA and PRIME Designation by the EMA, underscoring its potential as a groundbreaking therapy for Gaucher disease and possibly extending to genetically linked subsets of Parkinson’s disease patients with GBA1 mutations.
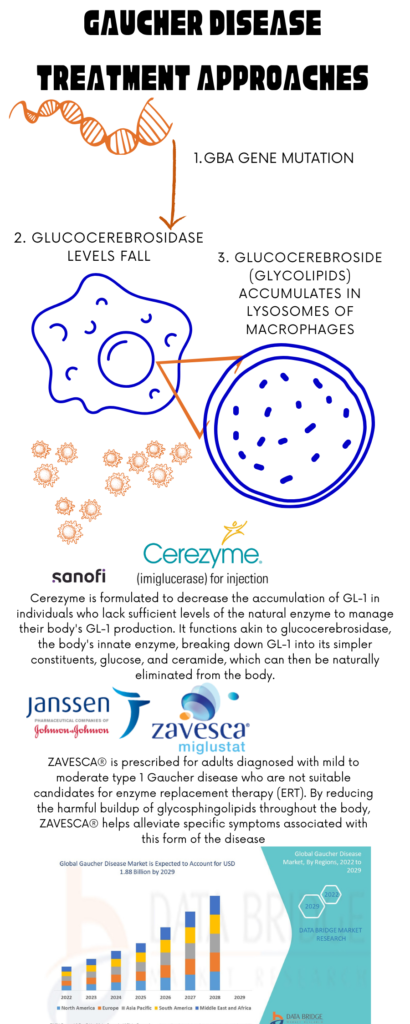
Image: Treatment Approaches for Gaucher Disease; Image Source: Malini Gupta
According to a report by Data Bridge Market Research, the Gaucher disease market was valued at USD 1.54 billion in 2021 and is anticipated to reach USD 1.88 billion by 2029, with a compound annual growth rate (CAGR) of 2.50% during the forecast period from 2022 to 2029. The comprehensive market report encompasses various insights including market segments, geographical coverage, key market players, and the overall market scenario. Additionally, it provides in-depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework to offer a comprehensive understanding of the market landscape and dynamics.
Overall, advancements in clinical trials offer promise for improved treatments and outcomes for those living with Gaucher disease.
Dr. Malini Gupta, Ph.D.
Sources
- https://www.zavesca.com/how-it-works
- https://www.cerezyme.com/
- https://gaucheralliance.org/clinical-trials/



