Diagnosing Erdheim-Chester Disease (ECD), presents significant challenges due to its rarity and varied clinical presentations. Successful diagnosis typically involves integrating descriptive pathology, clinical observations, and radiographic findings. Patients often undergo multiple biopsies, leading to delays in diagnosis and treatment initiation, with the average time from symptom onset to diagnosis ranging from months to years. Determining the appropriate treatment for ECD thus involves a collaborative effort between patients and their healthcare providers, while effective treatments exist, a cure has yet to be discovered. There are three main categories of typical ECD treatments: targeted therapies, chemotherapy, and immunotherapy (see Figure). Advances in genetic analysis now allow for the identification of various mutations in the BRAF and MAPK pathways, enabling the utilization of targeted therapies like vemurafenib, dabrafenib, or cobimetinib. Molecular testing plays a vital role in ECD diagnosis, as most patients exhibit activating somatic mutations or fusions in the MAPK-ERK or PI3K-AKT pathways. Molecular profiling of biopsy material can bolster diagnostic confidence, particularly in cases with unclear histopathological findings or lacking osseous lesions. However, some patients may not exhibit detectable driver alterations, or the biopsy sample may be inadequate for analysis. In such instances, stains like BRAF-VE1 or phosphorylated ERK can assist in diagnosis if notable cytoplasmic staining is present in lesional cells.
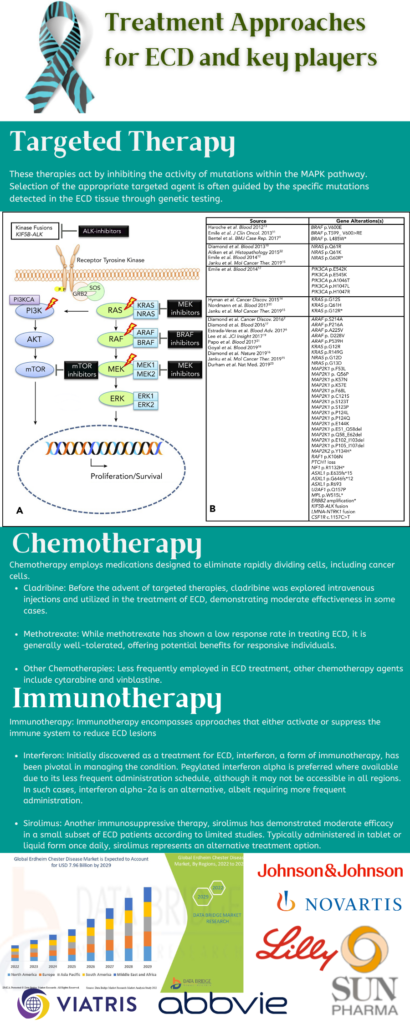
Figure 1: Treatment Approaches for ECD and key players, Image Source: Malini Gupta
One of the latest in the field includes a trial closed to accrual sponsored by Dana-Farber Cancer Institute (NCT02523040) headed by Eric Jacobsen, for the potential use of the chemotherapy drug Lenalidomide as a treatment option for one of three histiocytic disorders: Langerhans cell histiocytosis (LCH), Erdheim-Chester disease (ECD), or histiocytic sarcoma (HS). Lenalidomide, a drug categorized as an immunomodulatory drug (IMiD), has not received FDA approval for ECD yet, but it has been authorized for other medical purposes. As an IMiD, lenalidomide modulates the body’s immune response to combat cancer. It also has the potential to disrupt the formation of tiny blood vessels crucial for supporting tumor growth, thereby inhibiting, or impeding the proliferation of cancer cells. Lenalidomide’s efficacy lies in its ability to enhance the immune system’s capacity to target and eliminate tumor cells. In this phase II trial of studying the effect of Lenalidomide on Adult Histiocyte Disorders, over a period of 12 months, this trial is looking into : Progression Free Survival (PFS), Overall Survival, Number of Participating with Grade 3-4 toxicity, Quantitative serial measurements of urine, serum and plasma cell free DNA for BRAF mutation as a biomarker of response in patients over 16 years of age having histologically or cytologically confirmed Langerhans cell histiocytosis (LCH), Erdheim-Chester disease (ECD), or histiocytic sarcoma (HS). The dosage includes administration of lenalidomide orally (PO) from days 1 to 21, with treatment cycles repeating every 28 days for a total of 12 courses, provided there is no disease progression or intolerable side effects and dated for completion in August 2024.
Data Bridge Market Research forecasts a growth rate of approximately 7.10% in the global Erdheim-Chester disease market between 2022 and 2029. The market, valued at USD 4.6 billion in 2021, is projected to reach USD 7.96 billion by 2029.
Dr. Malini Gupta, Ph.D.
Sources
- https://erdheim-chester.org/news/2018-ecd-awareness-week/
- https://www.databridgemarketresearch.com/reports/global-erdheim-chester-disease-market
- https://ashpublications.org/view-large/figure/9676646/bloodBLD2019003507f1.tif
- https://clinicaltrials.gov/study/NCT02523040?cond=Erdheim-Chester%20Disease&aggFilters=status:rec%20com%20act&rank=8



