One of the most prevalent forms of muscle dystrophy affecting males, Duchenne Muscular Dystrophy (DMD) is a rare progressive muscle wasting disease which leads to challenges with mobility, progressive muscle weakness, respiratory difficulties, and ultimately premature death. There have been significant strides in gene-based treatments aimed at combating DMD as summarized in Figure 1. Among these, antisense-mediated exon skipping has emerged as a particularly hopeful strategy for tackling the muscular dystrophy associated with Duchenne. The latest to receive FDA approval though is the first of its kind nonsteroidal drug for treatment of DMD. Duvyzat (Givinostat), acts by inhibiting histone deacetylases (HDACs), enzymes responsible for altering the three-dimensional structure of DNA within cells, thereby impeding gene expression. A more expansive list of clinical trials and drug pipelines can be found in Cure Duchenne.

Figure I: Gene-Based Treatments Aimed at Combating DMD, Image Source: Malini Gupta
Duvyzat (Givinostat), an oral medication developed by Italfarmaco Group in collaboration with Lorenzo Puri and his team at Sanford Burnham Prebys Medical Research Institute, San Diego, along with partnerships with Telethon and Parent Project aps, has received FDA approval for treatment of patients with DMD in six year old onwards. Studies indicate that individuals with DMD often exhibit elevated HDAC activity, which may hinder muscle regeneration and incite inflammation. This drug “adds to list of approved treatments for families facing this devastating disease and is an important step forward in accelerating transformative treatments for everyone independent of their genetic mutation,” Debra Miller, founder, and CEO of CureDuchenne, said in a statement emailed to BioSpace.
Duvyzat’s efficacy in addressing DMD was evaluated in a phase 3 study spanning 18 months, employing a randomized, double-blind, placebo-controlled methodology. The primary gauge of effectiveness centered on the alteration in muscle function, assessed through a four-stair climb test from the study’s onset to the 18-month mark. Throughout the study, all participants adhered to the standard steroid regimen as part of their treatment protocol. At the culmination of the 18-month period, patients treated with Duvyzat displayed a statistically significant reduction in the time required to ascend four stairs compared to those administered placebo. Specifically, the mean change in climbing time from baseline to Month 18 was 1.25 seconds for patients receiving Duvyzat, as opposed to 3.03 seconds for those receiving placebo. Furthermore, a secondary measure of efficacy entailed evaluating alterations in physical function using the North Star Ambulatory Assessment (NSAA), a widely utilized scale for appraising motor function in ambulatory boys with DMD. The findings revealed that individuals treated with Duvyzat experienced lesser deterioration in their NSAA scores compared to those in the placebo group throughout the 18-month evaluation period. The FDA expedited the review process for this application, granting it priority review and fast track designation. Additionally, the treatment received orphan drug status and rare pediatric disease designations.
As of 2023, the Duchenne muscular dystrophy market held a value of USD 3.5 billion, experiencing notable expansion. Projections indicate that it is poised to reach USD 11.7 billion by 2033, showcasing a remarkable Compound Annual Growth Rate (CAGR) of 13.16%. Marketresearch.biz reports that advancements in genetic testing and screening are driving growth in the DMD market. Previously, families waited 2.2 to 2.3 years for a DMD diagnosis. But with improved diagnostic methods, including the detection of a distinct epigenetic signature, diagnoses in infants and children are now earlier and more precise.
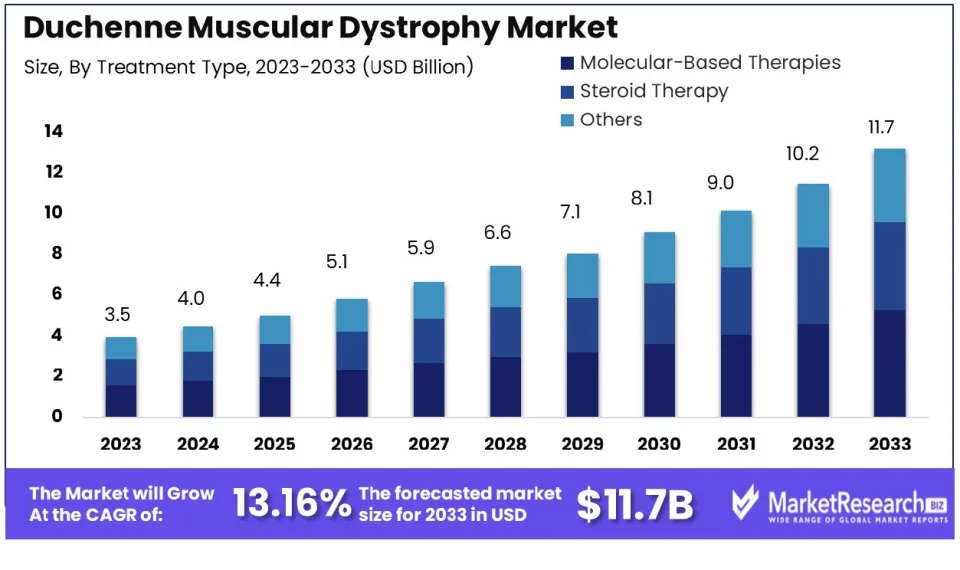
Figure 2: Duchenne Muscular Dystrophy (DMD) Treatment Type Market
Dr. Malini Gupta, Ph.D,
Sources
- https://www.fda.gov/news-events/press-announcements/fda-approves-nonsteroidal-treatment-duchenne-muscular-dystrophy
- https://www.nspharma.com/press-releases
- https://www.sarepta.com/science/gene-therapy-engine
- https://www.duchenne.com/considering-clinical-trials/drug-development-and-fda-approvalhttps://cureduchenne.org/
- https://marketresearch.biz/report/duchenne-muscular-dystrophy-market/



