What are rare diseases?
Being unique isn’t easy and Rare Diseases due to their low prevalence have not been an exception. In a law passed by the United States Congress in 1983 called the Orphan Drug Act, Rare Diseases are defined as conditions affecting less than 200,000 people in the United States. In contrast, the European Union designates Rare Diseases to affect 50 per 100,000 people. World Health Organization (WHO) on the other hand suggests a Rare Disease to be the one that can strike fewer than 65 per 100,000 people. Rare Diseases International (RDI) in a recent collaboration in 2019 with WHO has developed an “Internationally endorsed Operational Description of Rare Diseases” where “A rare disease is a medical condition with a specific pattern of clinical signs, symptoms, and findings that affects fewer than or equal to 1 in 2000 persons living in any region of the world”. Developing proper tools to categorize these diseases according to their occurrence, incidence, geographical relevance, and the number of people worldwide who are affected by them and building a repertoire for policymakers to have access to are elements included in the “Descriptive Framework” defined during this collaboration.
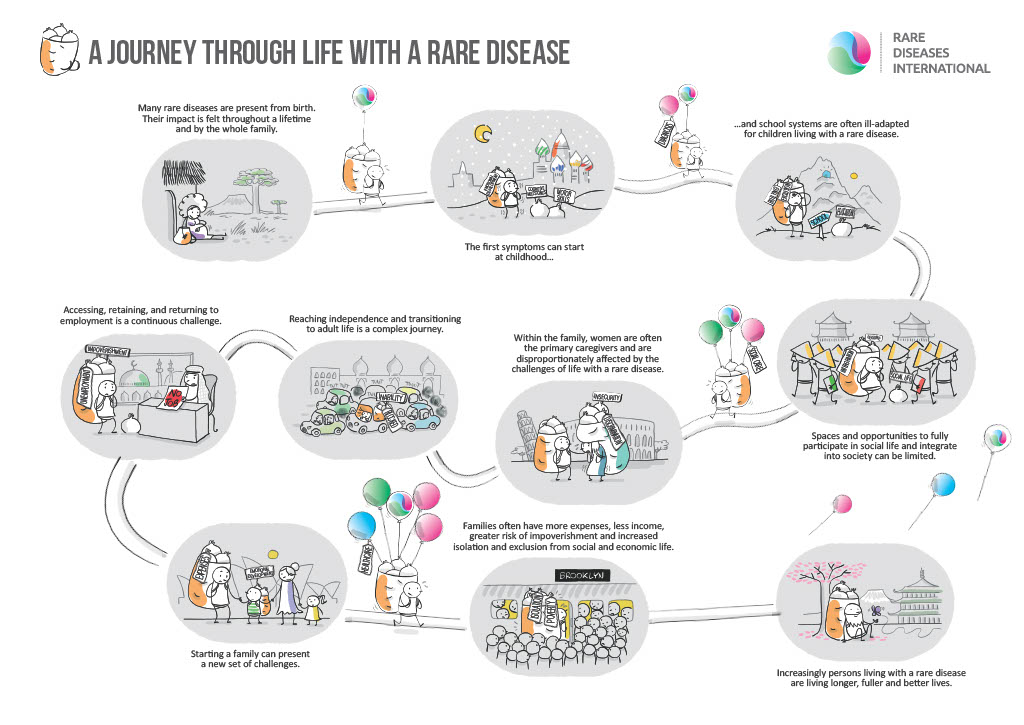
Image obtained from: https://www.rarediseasesinternational.org/living-with-a-rare-disease/
Why Care for Rare?
Approximately 80% of Rare Diseases stem from genetic origins, with nearly 70% manifesting during childhood. Shockingly, around 95% of these conditions currently lack approved treatments. The journey towards an accurate diagnosis typically spans an average of 4.8 years, and tragically, about 30% of children afflicted with a rare disease do not survive past the age of 5 years. Persons Living with a Rare Disease (PLWRD) represent a population of 300 million worldwide and they all struggle to obtain cures, therapies, proper care, and awareness. Lower case numbers and the prevalence of these diseases make it difficult to fund research and develop healthcare initiatives locally to help patients in need, or to spread awareness. Recognizing these pressing issues, the United Nations took a significant step in 2021 by adopting its first resolution to address the challenges faced by individuals living with rare diseases and their families urging member states to ensure access to safe and affordable healthcare services, particularly at the primary-care level.
Share for Rare: Collective knowledge and collaborations towards building a repertoire
Identifying Rare Diseases has been and still is tricky either due to overlapping symptoms with common conditions or presenting as apparently unrelated problems. So, the challenges posed by these ailments often lead from inefficient communication between patients and physicians, as well as limited literature and documentation restricting comprehensive care. “Orphanet”, established in 1997 in France and later supported by the European Commission, year 2000 onwards, aimed at scavenging and gathering knowledge on these conditions, to better diagnosis, care, and outcome of patients affected with Rare Diseases. It has currently grown to a network of 41 countries worldwide. This comprehensive platform not only contains an inventory of Rare Diseases but also an inventory of Orphan Drugs, a directory of expert clinics, laboratories, ongoing research, clinical trials, and the latest news in the field. The establishment of Clinical Research Networks (CRNs) for Rare Diseases also serves as a crucial focal point in addressing the challenges posed by small and geographically dispersed patient populations. These networks facilitate the thematic clustering of diseases and encourage collaborative efforts among various stakeholders, including patients, patient groups, researchers, healthcare providers, and industry professionals, as well as multidisciplinary teams. To promote a deeper understanding of the significance and functions of CRNs within the rare diseases community, the International Rare Diseases Research Consortium (IRDiRC) formed a Task Force intending to analyze the existing ecosystem and structure of national and international CRNs and pinpoint the barriers and requirements hindering international collaboration among these networks. Additionally, organizations and Non-Profits are working towards accumulating knowledge, resources, and advocacy worldwide include Rare Diseases International, EURORDIS, NCATS, and NORD, to name a few.
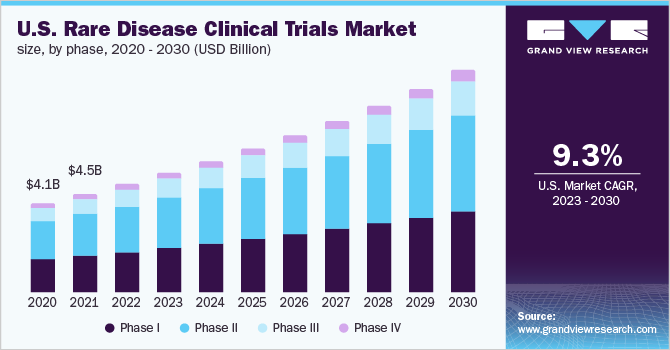
Image obtained from: https://www.grandviewresearch.com/industry-analysis/rare-disease-clinical-trials-market-report
Rare Necessities: Towards a brighter future?
Although there isn’t a consensus as to how many of these diseases have dedicated treatments, Rare Disease stakeholders suggest only 5% of these conditions have licensed treatments. From the perspective of drug development, the primary obstacle facing this sector remains striking a balance between funding research and development (R&D) while grappling with limited market opportunities due to small patient populations, resulting in constrained market sizes. Drug Repurposing by many companies is becoming increasingly popular in developing Orphan Drugs quickly and at a lower expense. In the United States, the 1983 Orphan Drug Act (ODA), established financial incentives for rare drug development including tax credits, waivers of Food and Drug Administration (FDA) user fees, and extensions of marketing exclusivity for rare indications. Also in the United States, a diverse array of programs now exists to mitigate risks and incentivize drug development for rare diseases such as voucher initiatives (e.g., for rare pediatric diseases), grant programs (e.g., established under the Rare Disease Act of 2002), Small Business Innovation grants/contracts, targeted research endeavors (e.g., Rare Cancer Moonshot), and various regulatory pathways (e.g., Accelerated Approval). In 2022, the National Institutes for Health (NIH) provided around $6.5 billion in funding for Rare Diseases and the FDA approved 20 orphan drugs out of 37 novel drugs (54%) and 9 orphan drugs granted fast-track approval (45%). Also in 2022, the global market size for clinical trials focused on Rare Diseases reached USD 11,455.0 million. Projections indicate a compound annual growth rate (CAGR) of 9.7% from 2023 to 2030. This expansion is driven by various factors, including the limited availability of drugs for Rare Diseases, advancements in personalized medicine, and the emergence of cell and gene therapies, all creating opportunities for developing new treatments. Additionally, heightened funding from pharmaceutical and biotechnology firms, as well as non-profit organizations, for Rare Disease clinical trials is contributing significantly to the market’s growth.
In this column, “Care for Rare”, Malini will take us on this journey to learn more about many of these Rare Diseases and the latest on the development of cures, with the hope of spreading more awareness.
Sources
What is a rare disease? – Rare Disease Day 2024 Day 2024
https://www.fda.gov/patients/rare-diseases-fda#whatisraredis
https://www.rarediseasesinternational.org/working-with-the-who/
https://www.thelancet.com/journals/langlo/article/PIIS2214-109X(24)00056-1/fulltext



