The field of liquid biopsy-based cancer diagnostics stands at an inflection point, primed to enable earlier detection, precise monitoring, and tailored cancer management. Myriad companies, predominantly startups but also including seasoned diagnostics players, are steering rapid innovations through blood tests that analyse circulating tumor DNA, cells, and other cancer biomarkers. Central to this transformation is circulating DNA, encompassing cell-free DNA (cfDNA), and its subset, circulating tumor DNA (ctDNA). This tandem has emerged as an unparalleled promise allied with the extraordinary strides in sequencing technologies. This multi-part series will chronicle the transformative thrusts of these diverse companies, explaining their distinguished histories, ground-breaking techniques, signature products, promising pipelines, and more. The companies take unique approaches yet share an overarching goal of early cancer detection. Their techniques leverage a diverse array of biomarkers, including genetic mutations, methylation markers, proteins, and inventive combinations thereof to discern cancer signals. Additionally, the burgeoning potential of machine learning algorithms to pinpoint subtle but indicative molecular patterns is gaining traction across the industry. Their offerings span early screening, residual disease tracking, and therapy selection. The series will also elucidate the field’s immense promise to profoundly impact patient outcomes through non-invasive assays that complement or even replace invasive tissue biopsies. However, liquid biopsy advances have also encountered unique challenges involving clinical adoption, reimbursement, regulatory approval, and alignment with therapeutics. The articles will investigate these hurdles and discuss how thoughtful research and flexible policies can pave the way for liquid biopsies to realize their paradigm-shifting potential. Overall, this series will illuminate the ground-breaking work of pioneering companies to transform cancer diagnostics with routine blood draws.
Overview
The landscape of in vitro diagnostics in cancer management stands on the precipice of transformation. It has long been evident that the most profound impact on cancer treatment lies in preemptive detection and intervention before clinical manifestation. Yet, in the absence of minimally intrusive and efficacious early screening techniques, this aspiration has remained mostly elusive. The tangible advantages of timely detection resonate in the statistics of the few malignancies to have recommended screening methods, such as breast, colorectal, lung, cervical, and prostate cancers. The last couple of decades have seen increasing recognition of assorted circulating agents as potential indicators of latent disease, ranging from DNA, proteins, metabolites, to membrane vesicles. Combining techniques for capturing and identifying these agents, with leveraging expansive datasets through data science, we are moving closer to the realization of the vision of early cancer diagnosis from routine blood samples. This progress is also giving rise to notable advancements and disruptions in the field of diagnostics.
Central to this transformation is circulating DNA, encompassing cell-free DNA (cfDNA), and its subset, circulating tumor DNA (ctDNA). This tandem has emerged as an unparalleled promise allied with the extraordinary strides in sequencing technologies. The dividends of decades of foundational research in cancer genetics and epigenetics are also manifesting in the form of discernible epigenetic changes and fragmentation patterns that offer the prospect of highly specific cancer characterization in combination with genetic alterations. Artificial intelligence (AI) also assumes significance, given importance of identifying health and disease related patterns with precision in the colossal datasets generated by these diagnostic methodologies. The merits of these assays extend beyond the early detection of nascent diseases across the diagnosis continuum, to encompass monitoring minimal residual disease (MRD), profiling tumors to tailor personalized therapies, and gauging treatment responses. The success of these will be pivotal in realizing the ambitious objectives of the Cancer Moonshot initiative. Launched in 2016 and revitalized in 2022 by President Biden, this initiative aspires to curtail cancer mortality rates by a 50% within the ensuing quarter-century.
Startups have consistently played a prominent role in advancing this field, maintaining leadership positions. Concurrently, well-established entities are strategically positioned to leverage the unfolding opportunities. Notable among the pioneering disruptors are companies such as GRAIL, Guardant Health, Natera, Exact Medicine, Foundation Medicine, and Personal Genome Diagnostics. While these entities exhibit certain shared attributes, each approach to early diagnosis has unique nuances. Over time, it is evident that there is significant convergence in the field toward a long elusive common objective: Multi Cancer Early Diagnosis (MCED).
GRAIL
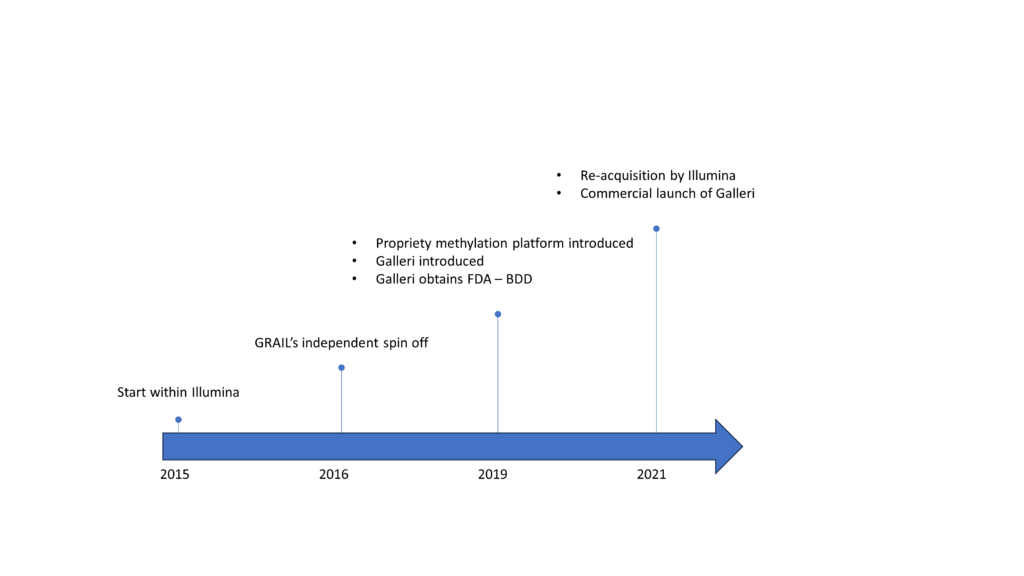
GRAIL had its beginning as an internal R&D unit within Illumina, catalyzed by the expanding recognition of cfDNA’s potential in cancer detection by about 2012. This set the stage for a dedicated team to focus on achieving the earliest possible detection and localization of cancer by analyzing methyl group patterns and mutations in cfDNA fragments found in the bloodstream. The team demonstrated its prowess in identifying cancer signals across various cancer types such as breast, colon, ovarian, and liver. However, the necessity for extensive clinical trials and regulatory approvals essential for commercial diagnostic tests prompted Illumina’s decision to establish an independent entity. This audacious endeavor, aptly named GRAIL, was initiated in 2016, bolstered by a significant $100 million Series A investment from Illumina and top Silicon Valley venture capital firms. GRAIL’s approach, methodical with its focus from the outset on the overarching and ambitious goal of MCED, gathered momentum following its separation from Illumina, adopting the agility of a startup.
By 2017, GRAIL had analyzed over 7,000 blood samples in its comprehensive Cell-Free Genome Atlas study. This meticulous endeavor yielded groundbreaking research articles that characterized ctDNA and identified methylation markers with the precision to discern the origin tissues of cancers. Another pivotal stride was the strategic acquisition of Cirina, a Hong Kong-based company specializing in cell-free DNA analysis. Founded by Dennis Lo, the pioneer in analysis of circulating fetal DNA in maternal blood, fusion of Cirina’s innovative methylation analysis technology with GRAIL’s capabilities emerged as a linchpin in the pursuit of early cancer detection. In 2019, GRAIL introduced its proprietary methylation technology that combines DNA sequencing and machine learning to decode cancer patterns by analyzing over a million methylation sites within cfDNA fragments.
In 2019, GRAIL introduced Galleri, a pioneering multi-cancer early detection test. Galleri harnessed high-throughput sequencing and machine learning algorithms trained on extensive datasets to identify over 50 cancer types from a standard blood draw and demonstrated significant capability to predict the tissue type or organ associated with the origin of the cancer signal (CSO) with high accuracy. The same year, Galleri secured FDA Breakthrough Device Designation (BDD) and GRAIL initiated the influential Pathfinder interventional study. Results published between 2021 and 2022 showcased Galleri’s impressive achievements with high specificity and sensitivities across multiple cancers. By 2020, GRAIL raised more than $2 billion over several rounds of funding and in a significant turn of events, GRAIL filed for a proposed initial public offering (IPO) by submitting a registration statement on Form S-1 to the Securities and Exchange Commission (SEC) in September 2020. However, the narrative took an unexpected twist as 2021 concluded with the news of Illumina reacquiring GRAIL for a substantial $8 billion. The deal triggered a protracted legal saga, contested by the US FTC and the EU anti-trust probe. Ongoing legal developments persist, compounded by Illumina’s management disruptions and a Securities and Exchange Commission investigation. The year 2021 also marked the commercial launch of Galleri.
As these legal developments continue to unfold, Galleri maintains its commercial rollout and GRAIL forges ahead with an extensive clinical study program, such as the prospective CCGA (The Circulating Cell-free Genome Atlas) study involving over 15,000 participants. Expected to yield results in 2024, CCGA will further refine GRAIL’s models through a comprehensive comparison of cancer and non-cancer cfDNA. If GRAIL’s vision prevails, its DNA scanning technology could eventually stand as a profound cancer advancement akin to mammography and colonoscopy—an accomplishment of monumental significance in a field where even modest gains can translate to lives saved. Notably, the recent results from the UK based Symplify study underscored the efficacy of GRAIL’s MCED methods, particularly in detecting and predicting CSO in patients presenting general symptoms unrelated to the organ of origin. The expansive STRIVE study, a prospective trial involving 100,000 women, aims to identify cancers at earlier stages than the current Galleri by employing advanced methylation analysis. Simultaneously, the prospective non-interventional REFLECTION study monitors more than 15,000 patients who underwent the Galleri test, while the ongoing PATHFINDER 2 evaluates the performance of GRAIL’s MCED techniques in a screening eligible population.
These endeavors are geared towards achieving comprehensive FDA approval, positioning GRAIL as the pioneer in fully FDA-approved MCED provider. Concurrently, GRAIL explores broader early diagnosis horizons via partnerships with Amgen, AstraZeneca, and Bristol Myers Squibb to assess methylation based MRD detection. Moreover, the insights shared during the 2023 American Association for Cancer Research (AACR) meeting underscored the effectiveness of GRAIL’s methylation platform across the cancer diagnosis continuum. These findings indicate a promising opportunity for the platform’s expansion into post-diagnostic research and clinical applications, reaffirming GRAIL’s enduring commitment to advancing cancer diagnosis through a remarkable journey, filled with innovation and challenges.