Treatment options for Beta Thalassemia involve alleviating symptoms, managing complications, and improving quality of life. The specific treatment approach depends on the type and severity of Thalassemia. Standard therapy for this disease includes repeated lifelong blood transfusions to compensate for the lack of enough functional red blood cells. Patients with transfusion-dependent Thalassemia (TDT)require transfusions every 3-4 weeks. This is often thus combined with iron chelator therapies, as a preventative against iron overload side effects. Non-transfusion therapy comprises a) drugs, which enhance the Hemoglobin levels to decrease blood transfusion dependence b) gene therapy: a promising evolving approach, involving genetically manipulating the patient’s hematopoietic stem cells for proper expression of Hemoglobin and transplanting it back into the patient.
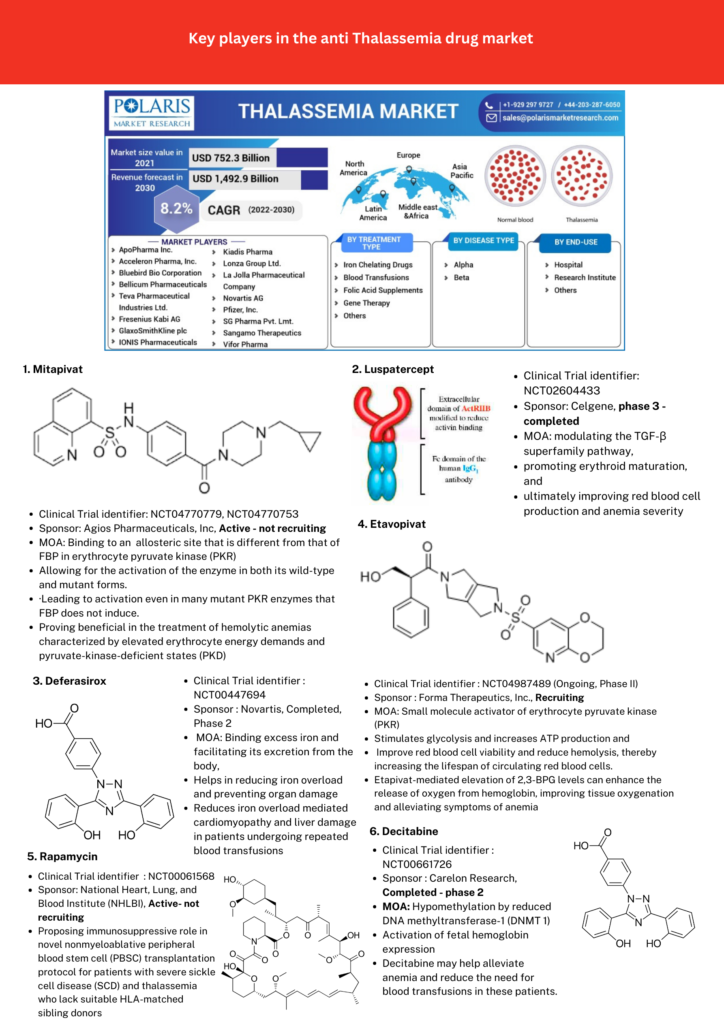
Promising candidate drugs in clinical trials with hopes of replacing or decreasing dependency on blood transfusion therapy are listed in Figure 1.
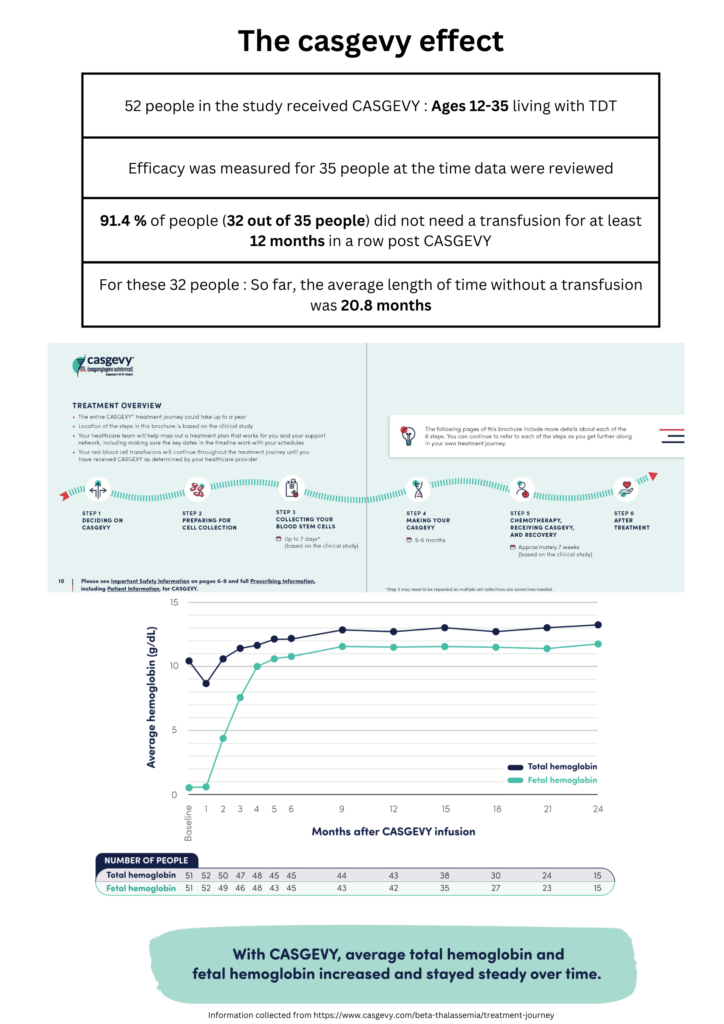
Gene therapy presents itself as a standalone alternative treatment option for TDT where the patient’s hematopoietic stem cells are manipulated to produce near-normal levels of Hemoglobin. Back in August 2022, beti-cel (brand name Zynteglo®), was the first potentially curative gene therapy for people with TDT approved by the FDA. The treatment had been manufactured by bluebird bio. In a more recent development by CRISPR Therapeutics, in collaboration with Vertex Pharmaceuticals, CASGEVY ™ (exagamglogene autotemcel [exa-cel]) has been approved by the FDA in January 2024, being the first ever FDA-approved genome editing treatment initially designated as a cure for sickle cell disease, then expanded to be used for the treatment of TDT in patients 12 years and older. CASGEVY is a non-viral ex vivo CRISPR/Cas9 gene therapy that offers a one-time treatment option for eligible patients and works by editing a patient’s own hematopoietic stem and progenitor cells at the erythroid-specific enhancer region of the BCL11A gene, leading to enhanced production of fetal hemoglobin (HbF). HbF is the form of the oxygen-carrying hemoglobin present during fetal development, which switches to the adult form of hemoglobin after birth. Since administering CASGEVY requires stem cell transplantation, Vertex Pharmaceuticals Incorporated has been working with experienced hospitals to establish a network of independently operated, authorized treatment centers (ATCs) throughout the U.S. to offer CASGEVY to patients with TDT and sickle cell disease (SCD).
Transparency Market Research predicts that by 2031, the global thalassemia treatment market will reach a value of US$1.5 billion, with a projected annual growth rate of 7.3%. With ongoing advancements in innovative treatments like gene editing therapies, stem cell transplants, and promising drug therapies in clinical trials, there’s optimism for reducing reliance on transfusions and improving the quality of life for patients and their families.
Malini Gupta, Ph.D.
Sources:
- Information for graphics obtained from:
- https://www.polarismarketresearch.com/industry-analysis/thalassemia-market
- https://www.casgevy.com/beta-thalassemia
- Beta-Thalassemia: A Pharmacological Drug-Based Treatment, Biswas et al, Drug and Drug Candidates, 2024.
- https://www.transparencymarketresearch.com/thalassemia-treatment-market.html



