On March 5th, 2024, Camurus AB, a Swedish biopharmaceutical company, announced FDA approval for the review of their New Drug Application (NDA) for OclaizTM (CAM2029) for the treatment of Acromegaly. The FDA has also set a Prescription Drug User Fee Act (PDUFA) target action date for October 21, 2024. The NDA submission is supported by data from seven clinical studies, including two Phase 3 trials within the ACROINNOVA program.
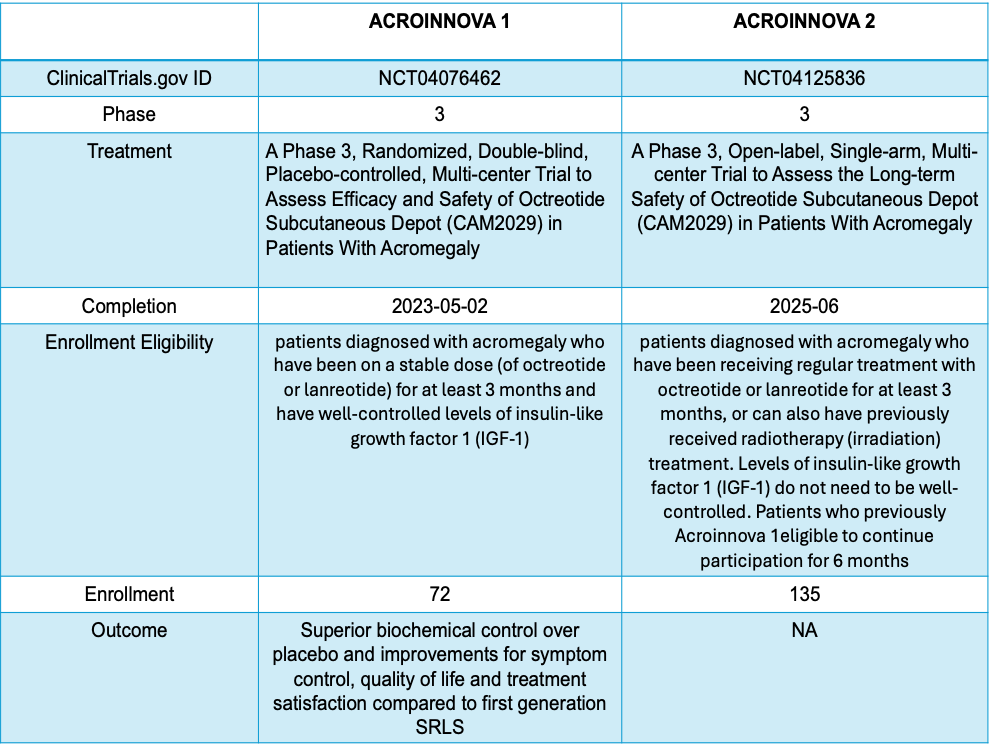
The Phase 3 studies in the ACROINNOVA program assess the efficacy and safety of octreotide subcutaneous (SC) depot, known as CAM2029. CAM2029 is designed for convenient, once-monthly self-administration, with enhanced octreotide plasma exposure and robust disease control.
Octreotide, a synthetic analog of GH inhibiting hormone, Somatostatin, binds to Somatostatin receptors on pituitary adenoma cells, inhibiting growth hormone (GH) release and normalizing elevated insulin-like growth factor-1 (IGF-1) levels in Acromegaly, thus improving associated symptoms and complications.
CAM2029 utilizes a novel formulation of injectable subcutaneous octreotide based on Fluid Crystal Injection depot technology. This technology employs a lipid drug delivery system that generates liquid crystal nanostructures in an aqueous environment, controlling the drug’s release rate. CAM2029 offers several advantages, including I) monthly subcutaneous administration ii) use of thin needles iii) compatibility with prefilled administration devices iv) stability at room temperature v) and significantly increased plasma exposure compared to octreotide LAR.
Findings from Acroinnova I showed that Octreotide SC depot is significantly more effective than a placebo in managing Acromegaly. This is evident from the higher rates of IGF-1 response (72.2% vs. 37.5%, p=0.0018) and a combined IGF-1 and GH response (70.0% vs. 37.5%, p=0.0035). Further analyses, including sensitivity and supportive assessments, confirm these results, showing a response rate of 81.0% for Octreotide SC depot compared to 38.1% for placebo in the per-protocol population (p=0.0002). Patients on Octreotide SC depot also experience prolonged response duration and well-maintained IGF-1 levels throughout the trial. Quality of life has been shown to improve significantly with Octreotide SC depot compared to standard care, with increased satisfaction in treatment convenience.
Post-completion of Acroinnova 1, patients have been given the choice of enrolling in Acroinnova 2, open-label treatment weeks 24-52 to assess the long-term safety and efficacy of CAM2029 in patients with Acromegaly who are to be administered CAM2029 subcutaneously once monthly for 12 months. In addition, a separate group of patients with active Acromegaly on treatment with first-generation SRLs with different degrees of biochemical response have also been included in this study, making a total of 135 patient enrollments. The study is expected to end in June 2025. Read the main story on Camurus press release.
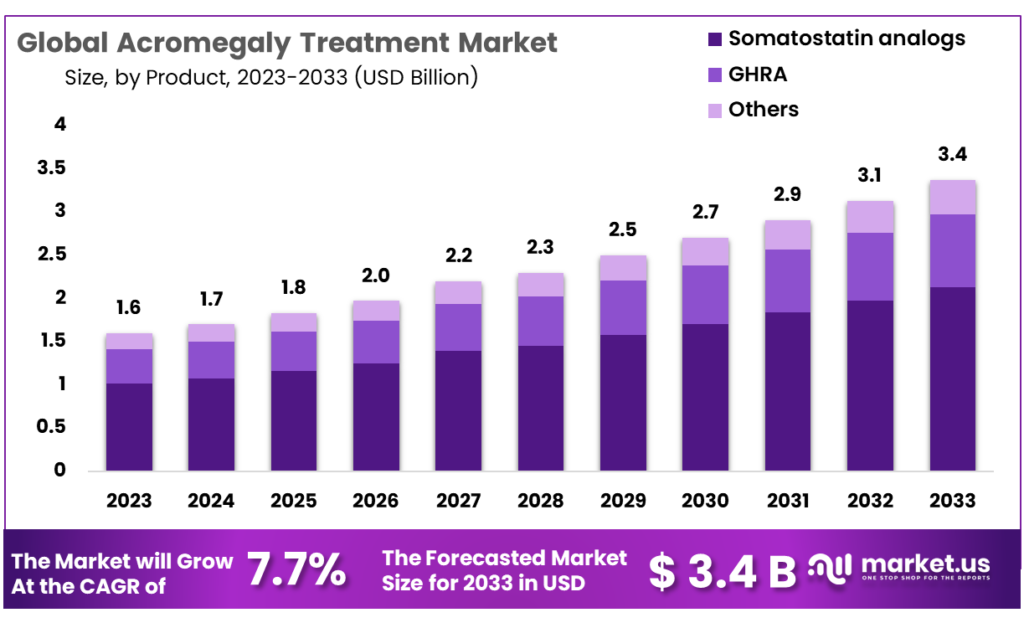

Additionally, according to market.us “Global Acromegaly Treatment Market size is expected to be worth around USD 3.4 Billion by 2033 from USD 1.6 Billion in 2023, growing at a CAGR of 7.7% during the forecast period from 2024 to 2033. Companies are now focusing on the implementation of strategies such as new formulations, partnerships, distribution agreements, and regional expansion to increase revenue share.”
Sources
- https://clinicaltrials.gov/study/NCT04125836?term=NCT04125836&rank=1
- https://clinicaltrials.gov/study/NCT04076462?term=NCT04076462&rank=1&tab=history&a=42
- New Treatments for Acromegaly in Development, Gadelha et al, The Journal of Clinical Endocrinology & Metabolism, 2024



