In a recent article published in the Cell Metabolism journal, scientists found that a combination of 12 gut microbes and 3 metabolites can predict the efficacy of anti-PD-1/PD-L1 therapy in various cancers with 88% sensitivity.
Need for the study: Immunotherapy against cancer has revolutionized cancer treatment and has now become the standard of care. There are various kinds of immunotherapies in use. One of these is immune checkpoint blockade therapy, which utilizes antibodies against immunosuppressors such as CTLA-4 (cytotoxic T-lymphocyte antigen 4), PD-1 (programmed cell death protein 1), and PD-L1 (programmed death ligand 1), either alone or in combination. Despite the promising effects of these therapies, only 20-40% of patients respond to these therapies. Thus, it is crucial to understand the potential molecular mechanism to improve the efficacy of these therapies or at least predict responders and non-responders.
Study details: A cohort of patients from various cancers undergoing anti-PD-1/PD-L1 immunotherapy (n=165) was used as a training cohort to determine differential fecal microbiome and metabolome between responders and non-responders. Additionally, four publicly available metagenomic datasets (n=568) were combined with the training cohort to determine the microbial and metabolic signatures. The findings from these cohorts were validated in an independent cohort (n=138). They found that 11 microbe species were enriched in responders and 6 in non-responders. These microbiomes predicted the responders with 78% sensitivity in the independent cohort. Similarly, they determined 9 metabolites with the highest predictive capability (79%). Combining both microbiome and metabolites, they found that 12 microbes and 3 metabolites predicted the responders to anti-PD-1/PD-L1 therapy with 88% sensitivity, performing better than microbe or metabolites alone.
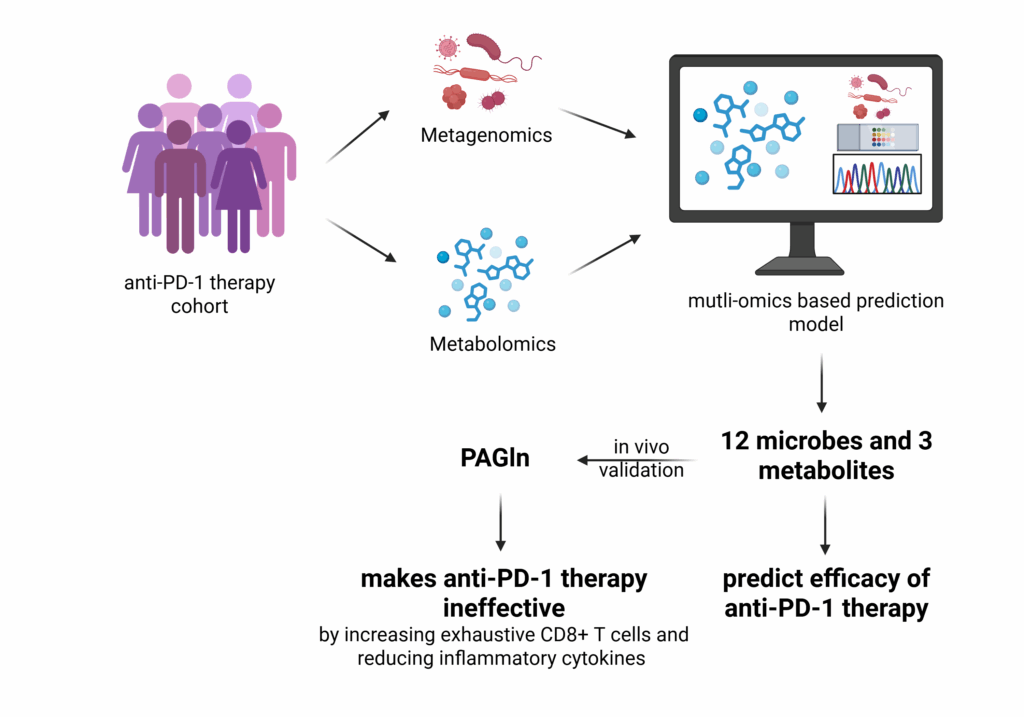
Figure: Multi-omics model shows gut microbiota and metabolites predicting anti-PD-1/PD-L1 immunotherapy efficacy, Image source: Vinny Negi
Potential mechanism of action
Among various metabolites detected, they found that only phenylacetylglutamine (PAGln) was negatively correlated with the anti-PD-1 therapy. Previous literature has shown that PAGln is produced by microbes by converting dietary phenylalanine and conjugating it with glutamine (mainly in humans) or glycine (mainly in rodents). They tested the role of PAGln in anti-PD-1 therapy in an animal model. Mice with tumors were given anti-PD-1 therapy in the absence and presence of PAGln. PAGln increased the tumor growth and also reduced the effect of anti-PD-1 therapy. Further analysis revealed an increased accumulation of exhausted CD8+ T cells in the presence of PAGln in the tumor microenvironment. PAGln also inhibited inflammatory cytokine synthesis such as IL-6 and TNF-α. Thereby, demonstrating the potential cellular and molecular mechanism of PAGln that reduces the response to anti-PD-1 therapy.
Study limitations:
- The sample size could limit the applicability of the findings to a diverse population.
- The genetic background of the animal model could have influenced the response. Thus, it needs to be validated in other species or strains.
- The threshold used for detecting metabolites might constrain the identification of subtle yet biologically relevant changes.
- The in vitro and in vivo models used may not fully recapitulate the human complexity.
–Dr. Vinny Negi, Ph.D.
Disclaimer
The editors take care to share authentic information. In case of any discrepancies please write to newsletter@medness.org
The sponsors do not have any influence on the nature or kind of the news/analysis reported in MedNess. The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of MedNess. Examples of analysis performed within this article are only examples. They should not be utilized in real-world analytic products as they are based only on very limited and dated open-source information. Assumptions made within the analysis are not reflective of the position of anyone volunteering or working for MedNess. This blog is strictly for news and information. It does not provide medical advice, diagnosis or treatment nor investment suggestions. This content is not intended to be a substitute for professional medical advice, diagnosis, or treatment. Always seek the advice of your physician or another qualified health provider with any questions you may have regarding a medical condition. Never disregard professional medical advice or delay in seeking it because of something you have read on this website.
MedNess is a part of STEMPeers® which is a 501(c)(3) organization registered in PA as PhD Career Support Group. The organization helps create a growing network of STEM scientists that is involved in peer-to-peer mentoring and support.



