X-linked protoporphyria (XLP) is a rare genetic disorder caused by mutations in the ALAS2 gene on the X chromosome. This gene encodes a protein called erythroid-specific 5-aminolevulinate synthase 2. Mutations in the ALAS2 lead to an overproduction of this enzyme, resulting in elevated levels of protoporphyrin IX. The excess protoporphyrin accumulates in the blood, liver, and skin, causing symptoms associated with the condition, including liver damage and the formation of gallstones.
Clinical Description, classification and symptoms
| Classification Level | Disorder |
| Prevalence | <1 / 1000,000 |
| Age of Onset | Childhood |
| Inheritance | X-linked dominant |
The hallmark of XLP is hypersensitivity of the skin to sunlight. People with this condition experience pain, itching, and a burning sensation after sun exposure, often accompanied by swelling and redness (erythema) in the affected areas. However, large blisters and severe scarring, which are common in other types of cutaneous porphyria, are not typical for XLP. Symptoms can appear within minutes of sun exposure, and although they usually subside within 24 to 48 hours, pain and a red or purple discoloration of the skin may last for several days. The pain is often disproportionately intense compared to the visible skin damage and can be excruciating, often unresponsive to pain medications, including narcotics. Repeated episodes of sun sensitivity may lead to long-term changes in the skin, such as thickening, hardening, a rough or leathery texture, small pock-like pits on the face, and grooving around the lips. In some cases, individuals with XLP may develop liver disease, which can range from mild liver dysfunction to complete liver failure. While information on liver disease in this condition is limited, the risk is thought to be higher in XLP compared to erythropoietic protoporphyria (EPP). Affected individuals may experience back pain and severe abdominal pain, especially in the upper right side of the abdomen. In some cases, bile flow through the gallbladder and bile ducts may be disrupted (cholestasis), leading to the formation of gallstones. These stones can cause blockages and inflammation of the gallbladder (cholecystitis). Liver scarring (cirrhosis) can also occur, and in some individuals, end-stage liver failure may eventually develop. Other symptoms reported in individuals with XLP include mild anemia (low red blood cell count) and iron deficiency.
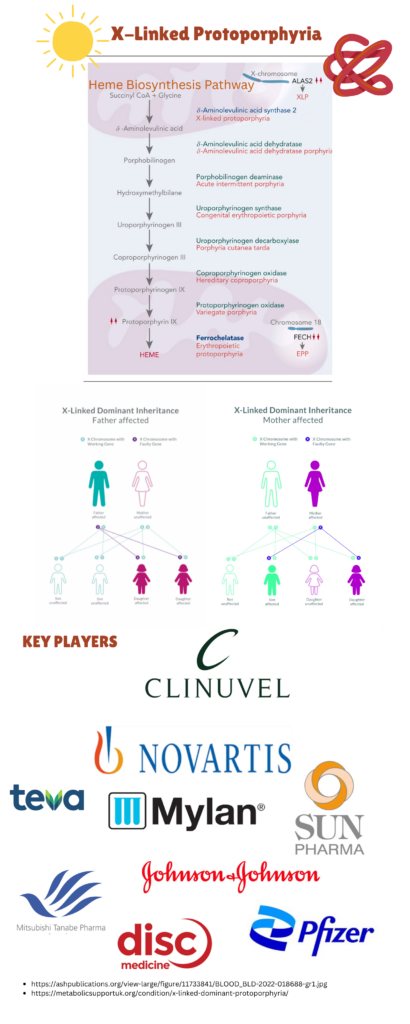
Image: Heme Biosynthesis Pathway of X-Linked Protoporphyria; Key players involved in the Treatment of Disease.
Diagnosis and Treatment
XLP is inherited in an X-linked pattern. Affected males pass the pathogenic variant to all their daughters and none of their sons. Females with an ALAS2 pathogenic variant have a 50% chance of passing the variant to each child. Once the ALAS2 variant is identified in an affected family member, testing for heterozygous female relatives, prenatal testing for at-risk pregnancies, and preimplantation genetic testing are available options. The diagnosis is confirmed in male as well as female proband by the detection of markedly elevated levels of free erythrocyte protoporphyrin and zinc-chelated erythrocyte protoporphyrin, along with the identification of a hemizygous pathogenic gain-of-function variant in the ALAS2 gene through molecular genetic testing.
Treatment options for XLP include Afamelanotide (Scenesse) to reduce photosensitivity, along with preventive measures like sunlight avoidance through protective clothing and tinted glass. Lumitene™ (β-carotene) may help some patients tolerate sunlight, though its effectiveness is not consistently proven. For liver complications, treatments like cholestyramine, plasmapheresis, and intravenous hemin may be used, with liver transplantation necessary in severe cases. Key companies involved in the research and treatment of XLP include Clinuvel Pharmaceuticals and Disc Medicine, where the latter is pursuing an accelerated approval for Bitopertin as a treatment for Erythropoietic Protoporphyria (EPP) and X-linked Protoporphyria, with a focus on protoporphyrin IX (PPIX) reduction as the surrogate endpoint. Bitopertin is an investigational GlyT1 inhibitor that modulates heme biosynthesis and could become the first disease-modifying therapy for erythropoietic porphyrias. They received FDA approval for the APOLLO post-marketing confirmatory trial, which will start by mid-2025.
For more on rare diseases, stay tuned for the next installment of Care for Rare. Meanwhile, click here for more available resources and support groups for XLP.
Dr. Malini Gupta, Ph.D.
Sources
- https://rarediseases.org/mondo-disease/x-linked-erythropoietic-protoporphyria/
- https://porphyriafoundation.org/
- https://www.omim.org/entry/300752
- https://www.databridgemarketresearch.com/reports/global-erythropoietic-protoporphyria-drugs-market
- https://www.discmedicine.com/news/disc-medicine-initiates-beacon-a-phase-2-clinical-study-of-bitopertin-in-patients-with-erythropoietic-protoporphyria-epp-and-x-linked-protoporphyria-xlp/
Disclaimer
The editors take care to share authentic information. In case of any discrepancies please write to newsletter@medness.org
The sponsors do not have any influence on the nature or kind of the news/analysis reported in MedNess. The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of MedNess. Examples of analysis performed within this article are only examples. They should not be utilized in real-world analytic products as they are based only on very limited and dated open-source information. Assumptions made within the analysis are not reflective of the position of anyone volunteering or working for MedNess. This blog is strictly for news and information. It does not provide medical advice, diagnosis or treatment nor investment suggestions. This content is not intended to be a substitute for professional medical advice, diagnosis, or treatment. Always seek the advice of your physician or another qualified health provider with any questions you may have regarding a medical condition. Never disregard professional medical advice or delay in seeking it because of something you have read on this website.
MedNess is a part of STEMPeers® which is a 501(c)(3) organization registered in PA as PhD Career Support Group. The organization helps create a growing network of STEM scientists that is involved in peer-to-peer mentoring and support.



