New Diabetic Drug Lixisenatide has found its new purpose to treat Parkinson disease. The studies published in New England Journal of Medicine suggested that lixisentide therapy attenuated the progression of motor disability in patients diagnosed with Parkinson disease in less than 3 years (Meissner et al., 2024). These benefits were associated with gastrointestinal side effects. Although, larger, longer, and comprehensively clinical trials are needed to assert/discern the benefits of lixsenatide.
Lixsenatide, is a glucagon -like peptide-1 receptor agonist. It is used for the treatment of diabetes. The trial of Lixsenatide in early Parkinson’s disease was a double-blind, randomized, placebo-controlled trial. 156 participants were randomly assigned. 78 was assigned to each group (treated and controlled). They received subcutaneous injection of Lixsenatide in addition to the regular Parkinson medication for one year. Lixsenatide or equivalent volume of placebo was administered at 10 ug per day for 14 days. The dose was increased to 20 ug per day for remaining one year.
The primary and secondary efficacy end points were based movement disorder society-unified Parkinson’s disease rating scale (MDS-UPDRS) scores. The clinical trials finding suggested reduction in motor disability progression in patients. Although, patients had gastrointestinal side effects, 46% participants had nausea and 13% experiences vomiting.
At baseline in both groups the score was 15. After one year, scores changed by -0.04 (improvement in moment) points in treatment group and 3.04 points (worsening of the disability) in the placebo group. Although, results relative to secondary end points did not differ between both groups.
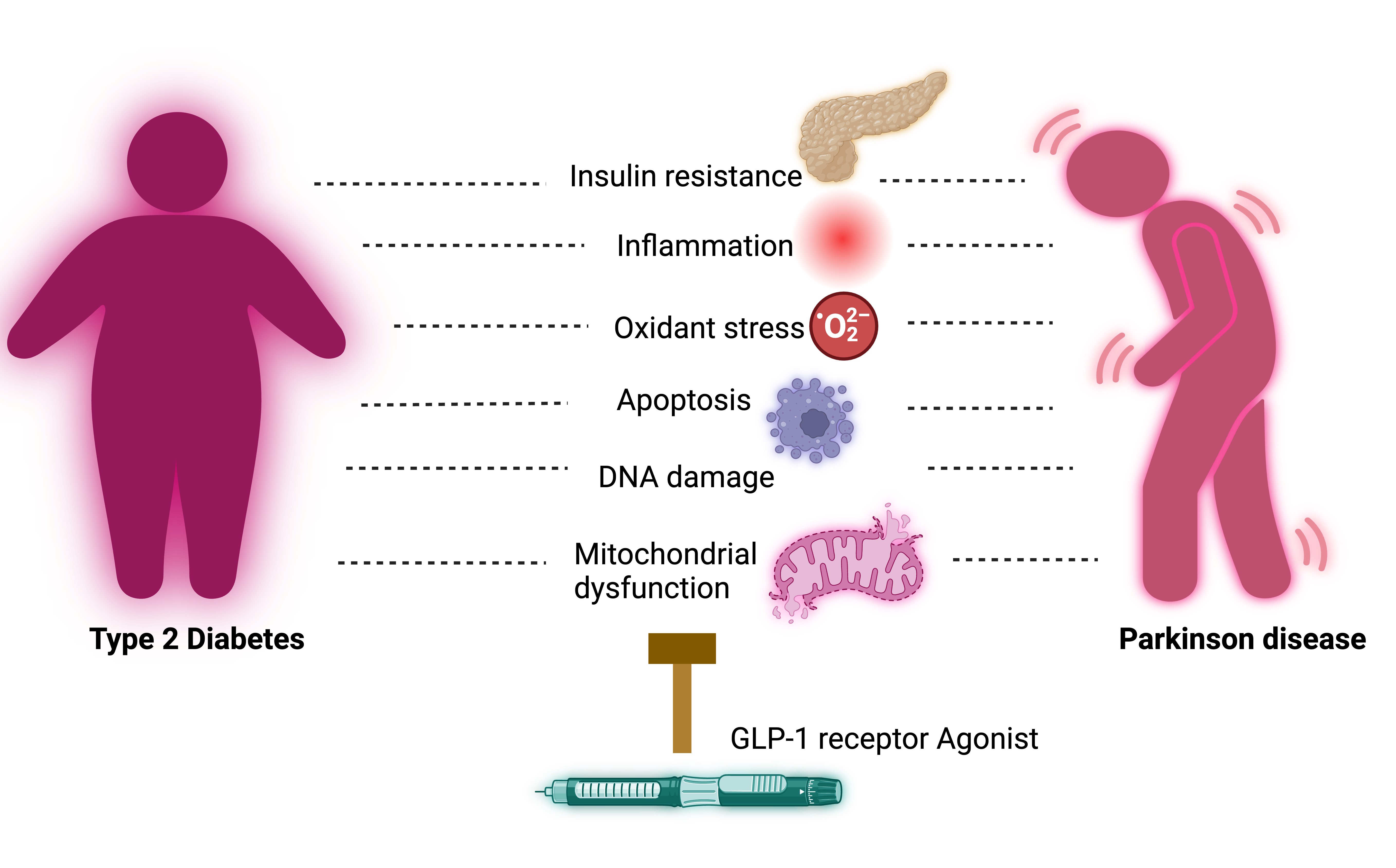
Illustration- GLP-1 receptor Agonist therapeutic effects on Parkinson Disease
The possible therapeutics effects of GLP-1 receptor agonist on progression of Parkinson disease could be possible because of reduction in inflammation, oxidant stress (illustration). These are comprehensively studied culprits in progression of Parkinson disease.
– Isha Sharma, Ph.D.
Reference:
- Meissner, W.G. et al. Trial of Lixisenatide in early Parkinson disease. N Engl J Med 2024; 390:1176-1185.



