Food and Drug Administration (FDA) approved ZepboundTM (Tirzepatide) for weight management in 2023. This twincertin compound is approved for controlling blood sugar in patients suffering from T2DM. Trizepatide is a precise amalgamation of the glucose-dependent insulinotropic polypeptide (GIP) and Glucagon-like peptide (GLP-1) receptors and has added advantage over Semaglutide, Liraglutide, Dulaglutide (agonist or activators of GLP1-R). Trizepatide has shown a considerable decrease in body weight, controlling blood sugar and insulin sensitivity, improving cardiac function, and another offshoot therapeutic effect is the attenuation of chronic kidney function (CKD). Trizepatide is a synthetic peptide of 39 amino acids that shares similarities with GIP (33 aa) and GLP-1 (30 aa). The difference is the addition of aminoisobutyric acid residue at 2, 13 position and 1,20-eicosanedioic acid as a linker. This slight tweaking and modification have produced exciting results for patients suffering from obesity. As mentioned by Dr. Leonard Glass, senior vice president of global medical affairs, Lilly Diabetes and Obesity-“Unfortunately, despite scientific evidence to the contrary, obesity is often seen as a lifestyle choice-something people should manage themselves” ( Lilly, 2023).
Infographic credit: Isha Sharma
The FDA approval results from phase SURMOUNT-1 and SURMOUNT-2 trials. In both studies, adults with obesity or its associated medical problems were included. The individuals taking Trizepatide along with a well-balanced diet and exercise have shown considerable weight loss compared to the placebo group. Adults taking 15 mg of Zepbound showed a 48 Ib decrease in body weight. Infect, Zepbound dose as minimum of 5 mg reduced 34Ib of body weight.
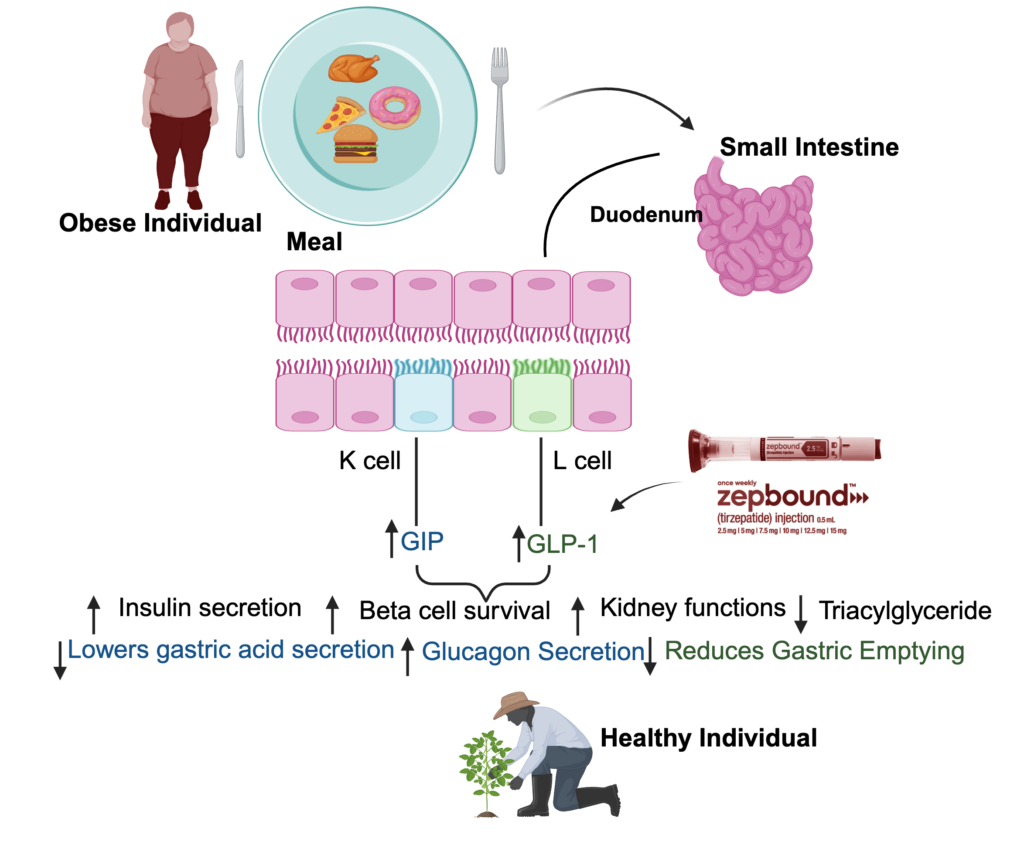
This wonder drug activates both GIPR and GLP1R. Trizepatide shares more similarities with GIP in its mechanism of action but differs from GLP1. GIP and GLP are hormones secreted by gut neuroendocrine cells once food enters the body. Their primary function is an increase in insulin secretion. They have another exciting and therapeutic function that has given them particular importance in the pharm industry, i.e., they both decrease appetite. Therefore, stimulating weight loss. GIP and GLP1 have a little antagonistic function in terms of glucagon secretion. GIP promotes it, and GLP1 diminishes its secretion. Meanwhile, GIP lowers gastric secretions, and GLP1 reduces gastric emptying. This retention of glucagon function gives Trizepatide an added advantage over other weight loss drugs like Semaglutide or Liraglutide.
Agonist for GIP receptors in lipocytes increases adipose tissue blood flow and stimulates lipid uptake. Zepbound has been shown to decrease apoprotein C-III (apoC-III) levels. Therefore, Trizepatide also helps lower triglycerides, improving cardiac health, a function utterly independent of reducing body weight.
The result of the SURPASS-2 clinical trial has clearly shown the superiority of Trizepatide over Semaglutide for obesity and T2DM. Although there was a difference in dose amount, Trizepatide was seven times higher than Semaglutide. The study showed considerable improvement in beta-cell function, insulin sensitivity, and glucagon function. The exciting fact about Trizepatide is that insulin sensitivity showed reduced branched-chain amino acids (BCAAs) expression. Moreover, BCAAs are known to aggravate endothelial dysfunction. Reduced expression of BCAAs is another benefit of Zepbound in that it helps in attenuating progression of CKD. The results of the SURPASS-4 random clinical trial showed the efficacy of Trizepatide in slowing the loss of eGFR (estimated glomerular filtration rate) as compared to insulin glargine (Bosch, 2023).
Given the potential benefits shown by Zepbound and the fact that it is the only weight loss drug approved by the FDA, patients and investors got excited about the rising stock value of Lilly. It is an infrequent phenomenon to see tremendous growth in the stock of a pharma company. However, Lilly stock climbed 117% in the past year (Climino, 2024). Zepbound not only benefited patients but the investors as well. Moreover, the company portfolio has improved considerably.
References:
- Bosch, C. Carriazo, S. Soler, M.J. Ortiz, A. Fernandez, B.F. (2023). Trizepatide and prevention of chronic kidney disease. Clinical Kidney Journal. 16(5), 797-808
- Cimino, A. (2024, Feb 12). Could Eli Lilly’s Zepbound Become the World’s Best-Selling Drug?. https://www.fool.com/investing/2024/02/12/could-lillys-zepbound-become-worlds-best-seller/#:~:text=Everyone%20is%20excited%20about%20Zepbound,117%25%20over%20the%20past%20year.
- Eli Lilly. (2023, November 8). FDA Approves Lilly’s Zepbound™ (tirzepatide) for Chronic Weight Management, a Powerful New Option for the Treatment of Obesity or Overweight with Weight-Related Medical Problems. https://investor.lilly.com/news-releases/news-release-details/fda-approves-lillys-zepboundtm-tirzepatide-chronic-weight



